A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells
- PMID: 10818150
- PMCID: PMC6772624
- DOI: 10.1523/JNEUROSCI.20-11-04145.2000
A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells
Abstract
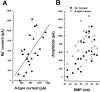
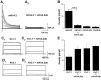
During neuronal differentiation and maturation, electrical excitability is essential for proper gene expression and the formation of synapses. The expression of ion channels is crucial for this process; in particular, voltage-gated K(+) channels function as the key determinants of membrane excitability. Previously, we reported that the A-type K(+) current (I(A)) and Kv4.2 K(+) channel subunit expression increased in cultured cerebellar granule cells with time. To examine the correlation between ion currents and the action potential, in the present study, we measured developmental changes of action potentials in cultured granule cells using the whole-cell patch-clamp method. In addition to an observed increment of I(A), we found that the Na(+) current also increased during development. The increase in both currents was accompanied by a change in the membrane excitability from the nonspiking type to the repetitive firing type. Next, to elucidate whether Kv4.2 is responsible for the I(A) and to assess the effect of Kv4 subunits on action potential waveform, we transfected a cDNA encoding a dominant-negative mutant Kv4.2 (Kv4.2dn) into cultured cells. Expression of Kv4.2dn resulted in the elimination of I(A) in the granule cells. This result demonstrates that members of the Kv4 subfamily are responsible for the I(A) in developing granule cells. Moreover, elimination of I(A) resulted in shortening of latency before the first spike generation. In contrast, expression of wild-type Kv4.2 resulted in a delay in latency. This indicates that appearance of I(A) is critically required for suppression of the excitability of granule cells during their maturation.
Figures








Similar articles
-
Elimination of the fast transient in superior cervical ganglion neurons with expression of KV4.2W362F: molecular dissection of IA.J Neurosci. 2000 Jul 15;20(14):5191-9. doi: 10.1523/JNEUROSCI.20-14-05191.2000. J Neurosci. 2000. PMID: 10884302 Free PMC article.
-
Molecular heterogeneity of the voltage-gated fast transient outward K+ current, I(Af), in mammalian neurons.J Neurosci. 2001 Oct 15;21(20):8004-14. doi: 10.1523/JNEUROSCI.21-20-08004.2001. J Neurosci. 2001. PMID: 11588173 Free PMC article.
-
Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes.J Physiol. 1999 Dec 15;521 Pt 3(Pt 3):587-99. doi: 10.1111/j.1469-7793.1999.00587.x. J Physiol. 1999. PMID: 10601491 Free PMC article.
-
What are the roles of the many different types of potassium channel expressed in cerebellar granule cells?Cerebellum. 2003;2(1):11-25. doi: 10.1080/14734220310015593. Cerebellum. 2003. PMID: 12882230 Review.
-
T-type channels buddy up.Pflugers Arch. 2014 Apr;466(4):661-75. doi: 10.1007/s00424-013-1434-6. Epub 2014 Jan 11. Pflugers Arch. 2014. PMID: 24413887 Free PMC article. Review.
Cited by
-
Relating ion channel expression, bifurcation structure, and diverse firing patterns in a model of an identified motor neuron.J Comput Neurosci. 2013 Apr;34(2):211-29. doi: 10.1007/s10827-012-0416-6. Epub 2012 Aug 11. J Comput Neurosci. 2013. PMID: 22878689 Free PMC article.
-
Fundamental Mechanisms of Autoantibody-Induced Impairments on Ion Channels and Synapses in Immune-Mediated Cerebellar Ataxias.Int J Mol Sci. 2020 Jul 13;21(14):4936. doi: 10.3390/ijms21144936. Int J Mol Sci. 2020. PMID: 32668612 Free PMC article. Review.
-
Quantitative interactions between the A-type K+ current and inositol trisphosphate receptors regulate intraneuronal Ca2+ waves and synaptic plasticity.J Physiol. 2013 Apr 1;591(7):1645-69. doi: 10.1113/jphysiol.2012.245688. Epub 2013 Jan 2. J Physiol. 2013. PMID: 23283761 Free PMC article.
-
Contribution of Kv4 channels toward the A-type potassium current in murine colonic myocytes.J Physiol. 2002 Oct 15;544(2):403-15. doi: 10.1113/jphysiol.2002.025163. J Physiol. 2002. PMID: 12381814 Free PMC article.
-
Voltage-gated potassium channel (Kv) subunits expressed in the rat cochlear nucleus.J Histochem Cytochem. 2008 May;56(5):443-65. doi: 10.1369/jhc.2008.950303. Epub 2008 Feb 5. J Histochem Cytochem. 2008. PMID: 18256021 Free PMC article.
References
-
- Arhem P, Johansson S. A model for the fast 4-aminopyridine effects on amphibian myelinated nerve fibres. A study based on voltage-clamp experiments. Acta Physiol Scand. 1989;137:53–61. - PubMed
-
- Baldwin TJ, Tsaur ML, Lopez GA, Jan YN, Jan LY. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. - PubMed
-
- Bardoni R, Belluzzi O. Kinetic study and numerical reconstruction of A-type current in granule cells of rat cerebellar slices. J Neurophysiol. 1993;69:2222–2231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
