Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy
- PMID: 10823860
- PMCID: PMC112040
- DOI: 10.1128/jvi.74.12.5542-5547.2000
Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy
Abstract
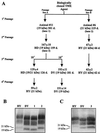
Interspecies transmission of the transmissible spongiform encephalopathies (TSEs), or prion diseases, can result in the adaptation and selection of TSE strains with an expanded host range and increased virulence such as in the case of bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease. To investigate TSE strain adaptation, we serially passaged a biological clone of transmissible mink encephalopathy (TME) into Syrian golden hamsters and examined the selection of distinct strain phenotypes and conformations of the disease-specific isoform of the prion protein (PrP(Sc)). The long-incubation-period drowsy (DY) TME strain was the predominate strain, based on the presence of its strain-specific PrP(Sc) following interspecies passage. Additional serial passages in hamsters resulted in the selection of the hyper (HY) TME PrP(Sc) strain-dependent conformation and its short incubation period phenotype unless the passages were performed with a low-dose inoculum (e.g., 10(-5) dilution), in which case the DY TME clinical phenotype continued to predominate. For both TME strains, the PrP(Sc) strain pattern preceded stabilization of the TME strain phenotype. These findings demonstrate that interspecies transmission of a single cloned TSE strain resulted in adaptation of at least two strain-associated PrP(Sc) conformations that underwent selection until one type of PrP(Sc) conformation and strain phenotype became predominant. To examine TME strain selection in the absence of host adaptation, hamsters were coinfected with hamster-adapted HY and DY TME. DY TME was able to interfere with the selection of the short-incubation HY TME phenotype. Coinfection could result in the DY TME phenotype and PrP(Sc) conformation on first passage, but on subsequent passages, the disease pattern converted to HY TME. These findings indicate that during TSE strain adaptation, there is selection of a strain-specific PrP(Sc) conformation that can determine the TSE strain phenotype.
Figures

References
-
- Bartz J C, McKenzie D I, Bessen R A, Marsh R F, Aiken J M. Transmissible mink encephalopathy species barrier effect between ferret and mink: PrP gene and protein analysis. J Gen Virol. 1994;75:2947–2953. - PubMed
-
- Bessen R A, Kocisko D A, Raymond G J, Nandan S, Lansbury P T, Caughey B. Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995;375:698–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

