Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism
- PMID: 10823899
- PMCID: PMC18764
- DOI: 10.1073/pnas.110035297
Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism
Abstract
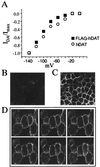
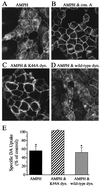
The dopamine transporter (DAT) is a target of amphetamine (AMPH) and cocaine. These psychostimulants attenuate DAT clearance efficiency, thereby increasing synaptic dopamine (DA) levels. Re-uptake rate is determined by the number of functional transporters at the cell surface as well as by their turnover rate. Here, we present evidence that DAT substrates, including AMPH and DA, cause internalization of human DAT, thereby reducing transport capacity. Acute treatment with AMPH reduced the maximal rate of [(3)H]DA uptake, decreased AMPH-induced currents, and significantly redistributed the immunofluorescence of an epitope-tagged DAT from the plasma membrane to the cytosol in human embryonic kidney 293 cells. Conversely, DAT inhibitors, such as cocaine, mazindol, and nomifensine, when administered with AMPH, blocked the reduction in [(3)H]DA uptake and the redistribution of DAT immunofluorescence to the cytosol. The reductions of [(3)H]DA uptake and AMPH-induced DAT internalization also were inhibited by coexpression of a dominant negative mutant of dynamin I (K44A), indicating that endocytosis modulates transport capacity, likely through a clathrin-mediated pathway. With this mechanism of regulation, acute application of AMPH would reduce DA uptake not only by direct competition for uptake, but also by reducing the available cell-surface DAT. Moreover, AMPH-induced internalization might diminish the amount of DAT available for DA efflux, thereby modulating the cytotoxic effects of elevated extracellular DA.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

