Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26
- PMID: 10823914
- PMCID: PMC18768
- DOI: 10.1073/pnas.120069197
Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26
Abstract
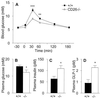
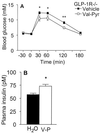
A subset of prolyl oligopeptidases, including dipeptidyl-peptidase IV (DPP IV or CD26, EC ), specifically cleave off N-terminal dipeptides from substrates having proline or alanine in amino acid position 2. This enzyme activity has been implicated in the regulation of the biological activity of multiple hormones and chemokines, including the insulinotropic peptides glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Targeted inactivation of the CD26 gene yielded healthy mice that have normal blood glucose levels in the fasted state, but reduced glycemic excursion after a glucose challenge. Levels of glucose-stimulated circulating insulin and the intact insulinotropic form of GLP-1 are increased in CD26(-/-) mice. A pharmacological inhibitor of DPP IV enzymatic activity improved glucose tolerance in wild-type, but not in CD26(-/-), mice. This inhibitor also improved glucose tolerance in GLP-1 receptor(-/-) mice, indicating that CD26 contributes to blood glucose regulation by controlling the activity of GLP-1 as well as additional substrates. These data reveal a critical role for CD26 in physiological glucose homeostasis, and establish it as a potential target for therapy in type II diabetes.
Figures



References
-
- De Meester I, Korom S, Van Damme J, Scharpe S. Immunol Today. 1999;20:367–375. - PubMed
-
- Morimoto C, Schlossman S F. Immunol Rev. 1998;161:55–70. - PubMed
-
- Kameoka J, Tanaka T, Nojima Y, Schlossman S F, Morimoto C. Science. 1993;261:466–469. - PubMed
-
- Marguet D, Bernard A M, Vivier I, Darmoul D, Naquet P, Pierres M. J Biol Chem. 1992;267:2200–2208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

