Cholesterol-dependent clustering of IL-2Ralpha and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts
- PMID: 10823948
- PMCID: PMC18550
- DOI: 10.1073/pnas.97.11.6013
Cholesterol-dependent clustering of IL-2Ralpha and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts
Abstract
Immunogold staining and electron microscopy show that IL-2 receptor alpha-subunits exhibit nonrandom surface distribution on human T lymphoma cells. Analysis of interparticle distances reveals that this clustering on the scale of a few hundred nanometers is independent of the presence of IL-2 and of the expression of the IL-2R beta-subunit. Clustering of IL-2Ralpha is confirmed by confocal microscopy, yielding the same average cluster size, approximately 600-800 nm, as electron microscopy. HLA class I and II and CD48 molecules also form clusters of the same size. Disruption of cholesterol-rich lipid rafts with filipin or depletion of membrane cholesterol with methyl-beta-cyclodextrin results in the blurring of cluster boundaries and an apparent dispersion of clusters for all four proteins. Interestingly, the transferrin receptor, which is thought to be located outside lipid rafts, exhibits clusters that are only 300 nm in size and are less affected by modifying the membrane cholesterol content. Furthermore, transferrin receptor clusters hardly colocalize with IL-2Ralpha, HLA, and CD48 molecules (crosscorrelation coefficient is 0.05), whereas IL-2Ralpha colocalizes with both HLA and CD48 (crosscorrelation coefficient is between 0.37 and 0.46). This coclustering is confirmed by electron microscopy. The submicron clusters of IL-2Ralpha chains and their coclustering with HLA and CD48, presumably associated with lipid rafts, could underlie the efficiency of signaling in lymphoid cells.
Figures


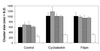



References
-
- Waldmann T A. Science. 1986;232:727–732. - PubMed
-
- Waldmann T A. J Biol Chem. 1991;266:2681–2684. - PubMed
-
- Nakamura Y, Russell S M, Mess S A, Friedmann M, Erdos M, Francois C, Jacques Y, Adelstein S, Leonard W J. Nature (London) 1994;369:330–333. - PubMed
-
- Damjanovich S, Gáspár R, Jr, Pieri C. Q Rev Biophys. 1997;30:67–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

