Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis
- PMID: 10823949
- PMCID: PMC18555
- DOI: 10.1073/pnas.97.11.6043
Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis
Abstract
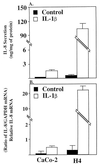
Necrotizing enterocolitis (NEC), a major cause of morbidity and mortality in premature infants, occurs after the introduction of oral feedings in conjunction with initial bacterial colonization of the gut and is hypothesized to be due to an immature (inappropriate) enterocyte response to bacterial stimuli. To test this hypothesis, we compared the enterocyte IL-8 response to inflammatory stimuli [lipopolysaccharide (LPS) and IL-1beta] in immature vs. mature human small intestine. Initial in vitro studies comparing confluent Caco-2 cells, a model for mature human enterocytes, with a primary human fetal intestinal cell line (H4 cells) demonstrated that after inflammatory stimulation fetal cells secreted more IL-8 (LPS, 8-fold; IL-1beta, 20-fold) than Caco-2 cells. IL-8 mRNA activity in fetal compared to Caco-2 cells was proportionately increased by the same magnitude with both stimuli. To validate the in vitro observations, small intestinal organ cultures from fetuses vs. older children were exposed to LPS and IL-1beta. Again in human organ cultures from fetuses compared to older children, IL-8 secretion was greater (LPS, 2.5-fold; IL-1beta, 200-fold) and mRNA activity after stimulation was comparably higher, suggesting that increased transcription of the IL-8 gene may account for the excessive response. Using immunohistochemical staining to identify the cellular source of IL-8, activity was noted predominantly in villous and crypt epithelium but also in a few immunoresponsive lymphoid cells. The observation that immature human enterocytes react with excessive pro-inflammatory cytokine production after inflammatory stimulation may help in part explain why prematures exposed to initial colonizing bacteria develop necrotizing enterocolitis.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

