Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain
- PMID: 10823959
- PMCID: PMC18579
- DOI: 10.1073/pnas.97.11.6185
Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain
Abstract
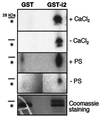
Signaling by the metabotropic glutamate receptor 1alpha (mGluR1alpha) can lead to the accumulation of inositol 1,4, 5-trisphosphate (InsP(3)) and cAMP and to the modulation of K(+) and Ca(2+) channel opening. At present, very little is known about how these different actions are integrated and eventually turned off. Unraveling the molecular mechanisms underlying these functions is crucial for understanding mGluR-mediated regulation of synaptic transmission. It has been shown that receptor-induced activation of the InsP(3) pathway is subject to feedback inhibition mediated by protein kinase C (PKC). In this study, we provide evidence for a differential regulation by PKC and protein kinase A of two distinct mGluR1alpha-dependent signaling pathways. PKC activation selectively inhibits agonist-dependent stimulation of the InsP(3) pathway but does not affect receptor signaling via cAMP. In contrast, protein kinase A potentiates agonist-independent signaling of the receptor via InsP(3). Furthermore, we demonstrate that the selectivity of PKC action on receptor signaling rests on phosphorylation of a threonine residue located in the G protein-interacting domain of the receptor. Modification at Thr(695) selectively disrupts mGluR1alpha-G(q/11) interaction without affecting signaling through G(s). Together, these data provide insight on the mechanisms by which selective down-regulation of a specific receptor-dependent signaling pathway can be achieved and on how cross-talk between different second messenger cascades may contribute to fine-tune short- and long-term receptor activity.
Figures
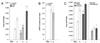


References
-
- Pin J-P, Duvoisin R M. Neuropharmacology. 1995;34:1–26. - PubMed
-
- Conn P J, Pin J-P. Annu Rev Pharmacol Toxicol. 1997;37:205–237. - PubMed
-
- Nakanishi S. Neuron. 1994;13:1031–1037. - PubMed
-
- Gudermann, N. C., Gahwiler, B. H. & Schultz, G. (1996) Annu. Rev. Pharmacol. Toxicol.429–459. - PubMed
-
- Clapham D E, Neer E J. Annu Rev Pharmacol Toxicol. 1997;37:167–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

