A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis
- PMID: 10823962
- PMCID: PMC18586
- DOI: 10.1073/pnas.97.11.6224
A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis
Abstract
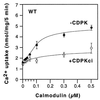
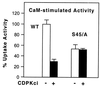
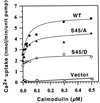
The magnitude and duration of a cytosolic Ca(2+) release can potentially be altered by changing the rate of Ca(2+) efflux. In plant cells, Ca(2+) efflux from the cytoplasm is mediated by H(+)/Ca(2+)-antiporters and two types of Ca(2+)-ATPases. ACA2 was recently identified as a calmodulin-regulated Ca(2+)-pump located in the endoplasmic reticulum. Here, we show that phosphorylation of its N-terminal regulatory domain by a Ca(2+)-dependent protein kinase (CDPK isoform CPK1), inhibits both basal activity ( approximately 10%) and calmodulin stimulation ( approximately 75%), as shown by Ca(2+)-transport assays with recombinant enzyme expressed in yeast. A CDPK phosphorylation site was mapped to Ser(45) near a calmodulin binding site, using a fusion protein containing the N-terminal domain as an in vitro substrate for a recombinant CPK1. In a full-length enzyme, an Ala substitution for Ser(45) (S45/A) completely blocked the observed CDPK inhibition of both basal and calmodulin-stimulated activities. An Asp substitution (S45/D) mimicked phosphoinhibition, indicating that a negative charge at this position is sufficient to account for phosphoinhibition. Interestingly, prior binding of calmodulin blocked phosphorylation. This suggests that, once ACA2 binds calmodulin, its activation state becomes resistant to phosphoinhibition. These results support the hypothesis that ACA2 activity is regulated as the balance between the initial kinetics of calmodulin stimulation and CDPK inhibition, providing an example in plants for a potential point of crosstalk between two different Ca(2+)-signaling pathways.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

