Mechanism of cGMP-gated channel block by intracellular polyamines
- PMID: 10828251
- PMCID: PMC2232895
- DOI: 10.1085/jgp.115.6.783
Mechanism of cGMP-gated channel block by intracellular polyamines
Abstract
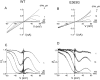
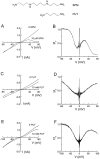
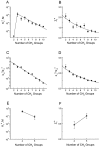
Polyamines block the retinal cyclic nucleotide-gated channel from both the intracellular and extracellular sides. The voltage-dependent mechanism by which intracellular polyamines inhibit the channel current is complex: as membrane voltage is increased in the presence of polyamines, current inhibition is not monotonic, but exhibits a pronounced damped undulation. To understand the blocking mechanism of intracellular polyamines, we systematically studied the endogenous polyamines as well as a series of derivatives. The complex channel-blocking behavior of polyamines can be accounted for by a minimal model whereby a given polyamine species (e.g., spermine) causes multiple blocked channel states. Each blocked state represents a channel occupied by a polyamine molecule with characteristic affinity and probability of traversing the pore, and exhibits a characteristic dependence on membrane voltage and cGMP concentration.
Figures


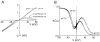




Similar articles
-
Mechanism of IRK1 channel block by intracellular polyamines.J Gen Physiol. 2000 Jun;115(6):799-814. doi: 10.1085/jgp.115.6.799. J Gen Physiol. 2000. PMID: 10828252 Free PMC article.
-
Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine.J Gen Physiol. 2005 Apr;125(4):413-26. doi: 10.1085/jgp.200409242. Epub 2005 Mar 14. J Gen Physiol. 2005. PMID: 15795311 Free PMC article.
-
Electrophysiological characteristics of rat gustatory cyclic nucleotide--gated channel expressed in Xenopus oocytes.J Neurophysiol. 2001 Jun;85(6):2335-49. doi: 10.1152/jn.2001.85.6.2335. J Neurophysiol. 2001. PMID: 11387380
-
Interactions of polyamines with ion channels.Biochem J. 1997 Jul 15;325 ( Pt 2)(Pt 2):289-97. doi: 10.1042/bj3250289. Biochem J. 1997. PMID: 9230104 Free PMC article. Review.
-
A fascinating tail: cGMP activation of aquaporin-1 ion channels.Trends Pharmacol Sci. 2002 Dec;23(12):558-62. doi: 10.1016/s0165-6147(02)02112-0. Trends Pharmacol Sci. 2002. PMID: 12457773 Review.
Cited by
-
Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels.J Gen Physiol. 2003 Nov;122(5):485-500. doi: 10.1085/jgp.200308890. J Gen Physiol. 2003. PMID: 14581581 Free PMC article.
-
Divergence of Ca(2+) selectivity and equilibrium Ca(2+) blockade in a Ca(2+) release-activated Ca(2+) channel.J Gen Physiol. 2014 Mar;143(3):325-43. doi: 10.1085/jgp.201311108. J Gen Physiol. 2014. PMID: 24567508 Free PMC article.
-
Stargazin and cornichon-3 relieve polyamine block of AMPA receptors by enhancing blocker permeation.J Gen Physiol. 2018 Jan 2;150(1):67-82. doi: 10.1085/jgp.201711895. Epub 2017 Dec 8. J Gen Physiol. 2018. PMID: 29222130 Free PMC article.
-
Ring of negative charge in BK channels facilitates block by intracellular Mg2+ and polyamines through electrostatics.J Gen Physiol. 2006 Aug;128(2):185-202. doi: 10.1085/jgp.200609493. Epub 2006 Jul 17. J Gen Physiol. 2006. PMID: 16847096 Free PMC article.
-
Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes.Br J Pharmacol. 2004 Dec;143(8):968-75. doi: 10.1038/sj.bjp.0706010. Epub 2004 Nov 22. Br J Pharmacol. 2004. PMID: 15557285 Free PMC article.
References
-
- Albert, A., and E.P. Serjeant. 1971. The determination of ionization constants (a laboratory manual). Chapman and Hall Ltd. London, UK.
-
- Araneda R.C., Zukin R.S., Bennett M.V.L. Effects of polyamines on NMDA-induced currents in rat hippocampal neuronsa whole-cell and single channel study. Neurosci. Lett. 1993;152:107–112. - PubMed

