Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130
- PMID: 10829066
- PMCID: PMC18633
- DOI: 10.1073/pnas.100135197
Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130
Abstract
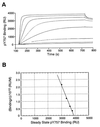
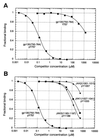
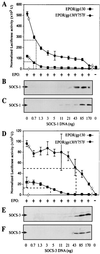
Suppressor of cytokine signaling-3 (SOCS-3) is one member of a family of intracellular inhibitors of signaling pathways initiated by cytokines that use, among others, the common receptor subunit gp130. The SH2 domain of SOCS-3 has been shown to be essential for this inhibitory activity, and we have used a quantitative binding analysis of SOCS-3 to synthetic phosphopeptides to map the potential sites of interaction of SOCS-3 with different components of the gp130 signaling pathway. The only high-affinity ligand found corresponded to the region of gp130 centered around phosphotyrosine-757 (pY757), previously shown to be a docking site for the tyrosine phosphatase SHP-2. By contrast, phosphopeptides corresponding to other regions within gp130, Janus kinase, or signal transducer and activator of transcription proteins bound to SOCS-3 with weak or undetectable affinity. The significance of pY757 in gp130 as a biologically relevant SOCS-3 docking site was investigated by using transfected 293T fibroblasts. Although SOCS-3 inhibited signaling in cells transfected with a chimeric receptor containing the wild-type gp130 intracellular domain, inhibition was considerably impaired for a receptor carrying a Y-->F point mutation at residue 757. Taken together, these data suggest that the mechanism by which SOCS-3 inhibits the gp130 signaling pathway depends on recruitment to the phosphorylated gp130 receptor, and that some of the negative regulatory roles previously attributed to the phosphatase SHP-2 might in fact be caused by the action of SOCS-3.
Figures

References
-
- Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

