Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants
- PMID: 10829075
- PMCID: PMC18631
- DOI: 10.1073/pnas.120067297
Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants
Abstract
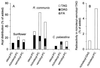
Triacylglycerol (TAG) is known to be synthesized in a reaction that uses acyl-CoA as acyl donor and diacylglycerol (DAG) as acceptor, and which is catalyzed by the enzyme acyl-CoA:diacylglycerol acyltransferase. We have found that some plants and yeast also have an acyl-CoA-independent mechanism for TAG synthesis, which uses phospholipids as acyl donors and DAG as acceptor. This reaction is catalyzed by an enzyme that we call phospholipid:diacylglycerol acyltransferase, or PDAT. PDAT was characterized in microsomal preparations from three different oil seeds: sunflower, castor bean, and Crepis palaestina. We found that the specificity of the enzyme for the acyl group in the phospholipid varies between these species. Thus, C. palaestina PDAT preferentially incorporates vernoloyl groups into TAG, whereas PDAT from castor bean incorporates both ricinoleoyl and vernoloyl groups. We further found that PDAT activity also is present in yeast microsomes. The substrate specificity of this PDAT depends on the head group of the acyl donor, the acyl group transferred, and the acyl chains of the acceptor DAG. The gene encoding the enzyme was identified. The encoded PDAT protein is related to lecithin:cholesterol acyltransferase, which catalyzes the acyl-CoA-independent synthesis of cholesterol esters. However, budding yeast PDAT and its relatives in fission yeast and Arabidopsis form a distinct branch within this protein superfamily, indicating that a separate PDAT enzyme arose at an early point in evolution.
Figures

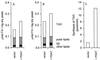
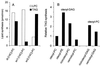

References
-
- Bell R M, Coleman R A. Annu Rev Biochem. 1980;49:459–487. - PubMed
-
- Stymne S, Stobart K. In: The Biochemistry of Plants: A Comprehensive Treatise. Stumpf P K, editor. Vol. 9. New York: Academic; 1987. pp. 175–214.
-
- Hobbs D H, Lu C, Hills M J. FEBS Lett. 1999;452:145–149. - PubMed
-
- Routaboul J M, Benning C, Bechtold N, Caboche M, Lepiniec L. Plant Physiol Biochem. 1999;37:831–840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

