Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice
- PMID: 10829080
- PMCID: PMC18747
- DOI: 10.1073/pnas.120166397
Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice
Abstract
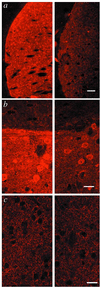
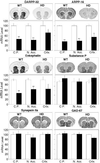
In Huntington's disease (HD), mutation of huntingtin causes selective neurodegeneration of dopaminoceptive striatal medium spiny neurons. Transgenic HD model mice that express a portion of the disease-causing form of human huntingtin develop a behavioral phenotype that suggests dysfunction of dopaminergic neurotransmission. Here we show that presymtomatic mice have severe deficiencies in dopamine signaling in the striatum. These include selective reductions in total levels of dopamine- and cAMP-regulated phosphoprotein, M(r) 32 kDA (DARPP-32) and other dopamine-regulated phosphoprotein markers of medium spiny neurons. HD mice also show defects in dopamine-regulated ion channels and in the D(1) dopamine/DARPP-32 signaling cascade. These presymptomatic defects may contribute to HD pathology.
Figures



References
-
- Gusella J F, MacDonald M E. Semin Cell Biol. 1995;6:21–28. - PubMed
-
- Vonsattel J P, Myers R H, Stevens T J J R, Ferrante E D B, Richardson E P. J Neurol Exp Neuropathol. 1985;44:559–577. - PubMed
-
- Roos R A C. In: Handbook of Clinical Neurology. Vinke P J, Bruyn G W, Klawanm H L, editors. Vol. 49. Amsterdam: Elsevier; 1986. pp. 315–327.
-
- Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
-
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S, Bates G P. Cell. 1996;87:493–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

