Molecular cloning, sequencing, and expression of omp-40, the gene coding for the major outer membrane protein from the acidophilic bacterium Thiobacillus ferrooxidans
- PMID: 10831405
- PMCID: PMC110521
- DOI: 10.1128/AEM.66.6.2318-2324.2000
Molecular cloning, sequencing, and expression of omp-40, the gene coding for the major outer membrane protein from the acidophilic bacterium Thiobacillus ferrooxidans
Abstract
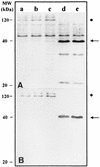
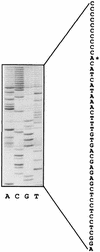
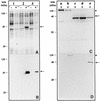
Thiobacillus ferrooxidans is one of the chemolithoautotrophic bacteria important in industrial biomining operations. Some of the surface components of this microorganism are probably involved in adaptation to their acidic environment and in bacterium-mineral interactions. We have isolated and characterized omp40, the gene coding for the major outer membrane protein from T. ferrooxidans. The deduced amino acid sequence of the Omp40 protein has 382 amino acids and a calculated molecular weight of 40,095.7. Omp40 forms an oligomeric structure of about 120 kDa that dissociates into the monomer (40 kDa) by heating in the presence of sodium dodecyl sulfate. The degree of identity of Omp40 amino acid sequence to porins from enterobacteria was only 22%. Nevertheless, multiple alignments of this sequence with those from several OmpC porins showed several important features conserved in the T. ferrooxidans surface protein, such as the approximate locations of 16 transmembrane beta strands, eight loops, including a large external L3 loop, and eight turns which allowed us to propose a putative 16-stranded beta-barrel porin structure for the protein. These results together with the previously known capacity of Omp40 to form ion channels in planar lipid bilayers strongly support its role as a porin in this chemolithoautotrophic acidophilic microorganism. Some characteristics of the Omp40 protein, such as the presence of a putative L3 loop with an estimated isoelectric point of 7.21 allow us to speculate that this can be the result of an adaptation of the acidophilic T. ferrooxidans to prevent free movement of protons across its outer membrane.
Figures



Similar articles
-
Phosphate starvation affects the synthesis of outer membrane proteins in Thiobacillus ferrooxidans.FEMS Microbiol Lett. 1992 Nov 1;77(1-3):29-33. doi: 10.1016/0378-1097(92)90127-a. FEMS Microbiol Lett. 1992. PMID: 1459418
-
Cloning and characterization of the gene encoding for OMP-PD porin: the major Photobacterium damsela outer membrane protein.Curr Microbiol. 2004 Mar;48(3):167-74. doi: 10.1007/s00284-003-4111-8. Curr Microbiol. 2004. PMID: 15057460
-
Porin isolated from the outer membrane of Erwinia amylovora and its encoding gene.Curr Microbiol. 2007 Feb;54(2):155-61. doi: 10.1007/s00284-006-0368-z. Epub 2007 Jan 5. Curr Microbiol. 2007. PMID: 17211539
-
The mer operon of the acidophilic bacterium Thiobacillus T3.2 diverges from its Thiobacillus ferrooxidans counterpart.Extremophiles. 1999 Jan;3(1):35-43. doi: 10.1007/s007920050097. Extremophiles. 1999. PMID: 10086843
-
Molecular genetics of Thiobacillus ferrooxidans.Microbiol Rev. 1994 Mar;58(1):39-55. doi: 10.1128/mr.58.1.39-55.1994. Microbiol Rev. 1994. PMID: 8177170 Free PMC article. Review.
Cited by
-
Integrative Genomics Sheds Light on Evolutionary Forces Shaping the Acidithiobacillia Class Acidophilic Lifestyle.Front Microbiol. 2022 Feb 15;12:822229. doi: 10.3389/fmicb.2021.822229. eCollection 2021. Front Microbiol. 2022. PMID: 35242113 Free PMC article.
-
Cytoplasmic CopZ-Like Protein and Periplasmic Rusticyanin and AcoP Proteins as Possible Copper Resistance Determinants in Acidithiobacillus ferrooxidans ATCC 23270.Appl Environ Microbiol. 2015 Dec 4;82(4):1015-1022. doi: 10.1128/AEM.02810-15. Print 2016 Feb 15. Appl Environ Microbiol. 2015. PMID: 26637599 Free PMC article.
-
Eurypsychrophilic acidophiles: From (meta)genomes to low-temperature biotechnologies.Front Microbiol. 2023 Mar 15;14:1149903. doi: 10.3389/fmicb.2023.1149903. eCollection 2023. Front Microbiol. 2023. PMID: 37007468 Free PMC article. Review.
-
Molecular basis of bacterial outer membrane permeability revisited.Microbiol Mol Biol Rev. 2003 Dec;67(4):593-656. doi: 10.1128/MMBR.67.4.593-656.2003. Microbiol Mol Biol Rev. 2003. PMID: 14665678 Free PMC article. Review.
-
Molecular aspects of bacterial pH sensing and homeostasis.Nat Rev Microbiol. 2011 May;9(5):330-43. doi: 10.1038/nrmicro2549. Epub 2011 Apr 5. Nat Rev Microbiol. 2011. PMID: 21464825 Free PMC article. Review.
References
-
- Aiba H, Adhya S, Combruggle B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. - PubMed
-
- Bainbridge G, Mobasheri H, Armstrong G A, Lea E J A, Lakey J H. Voltage-gating of Escherichia coli porin: a cystine-scanning mutagenesis study of loop 3. J Mol Biol. 1998;275:171–176. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

