Bacterial community structure and physiological state within an industrial phenol bioremediation system
- PMID: 10831417
- PMCID: PMC110543
- DOI: 10.1128/AEM.66.6.2400-2407.2000
Bacterial community structure and physiological state within an industrial phenol bioremediation system
Abstract
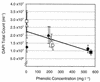
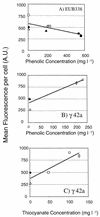
The structure of bacterial populations in specific compartments of an operational industrial phenol remediation system was assessed to examine bacterial community diversity, distribution, and physiological state with respect to the remediation of phenolic polluted wastewater. Rapid community fingerprinting by PCR-based denaturing gradient gel electrophoresis (DGGE) of 16S rDNA indicated highly structured bacterial communities residing in all nine compartments of the treatment plant and not exclusively within the Vitox biological reactor. Whole-cell targeting by fluorescent in situ hybridization with specific oligonucleotides (directed to the alpha, beta and gamma subclasses of the class Proteobacteria [alpha-, beta-, and gamma-Proteobacteria, respectively], the Cytophaga-Flavobacterium group, and the Pseudomonas group) tended to mirror gross changes in bacterial community composition when compared with DGGE community fingerprinting. At the whole-cell level, the treatment compartments were numerically dominated by cells assigned to the Cytophaga-Flavobacterium group and to the gamma-Proteobacteria. The alpha subclass Proteobacteria were of low relative abundance throughout the treatment system whilst the beta subclass of the Proteobacteria exhibited local dominance in several of the processing compartments. Quantitative image analyses of cellular fluorescence was used as an indicator of physiological state within the populations probed with rDNA. For cells hybridized with EUB338, the mean fluorescence per cell decreased with increasing phenolic concentration, indicating the strong influence of the primary pollutant upon cellular rRNA content. The gamma subclass of the Proteobacteria had a ribosome content which correlated positively with total phenolics and thiocyanate. While members of the Cytophaga-Flavobacterium group were numerically dominant in the processing system, their abundance and ribosome content data for individual populations did not correlate with any of the measured chemical parameters. The potential importance of the gamma-Proteobacteria and the Cytophaga-Flavobacteria during this bioremediation process was highlighted.
Figures




References
-
- Ahamad P Y A, Kunhi A A M. Degradation of phenol through ortho-cleavage pathway by Pseudomonas stutzeri strain SPC2. Lett Appl Microbiol. 1996;22:26–29.
-
- Bailey M J. Extraction of DNA from the phylosphere. In: Trevors J, Van Elsas J D, editors. Nucleic acids in the environment. Berlin, Germany: Springer-Verlag; 1995. pp. 89–109.
-
- Bandyopadhyay K, Das D, Maiti B R. Kinetics of phenol degradation using Pseudomonas putida MTCC 1194. Bioprocess Eng. 1998;18:373–377.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

