Characterization of a bifunctional enzyme fusion of trehalose-6-phosphate synthetase and trehalose-6-phosphate phosphatase of Escherichia coli
- PMID: 10831428
- PMCID: PMC110565
- DOI: 10.1128/AEM.66.6.2484-2490.2000
Characterization of a bifunctional enzyme fusion of trehalose-6-phosphate synthetase and trehalose-6-phosphate phosphatase of Escherichia coli
Abstract
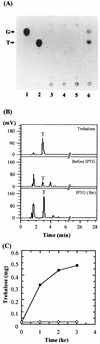
To test the effect of the physical proximity of two enzymes catalyzing sequential reactions, a bifunctional fusion enzyme, TPSP, was constructed by fusing the Escherichia coli genes for trehalose-6-phosphate (T6P) synthetase (TPS) and trehalose-6-phosphate phosphatase (TPP). TPSP catalyzes the sequential reaction in which T6P is formed and then dephosphorylated, leading to the synthesis of trehalose. The fused chimeric gene was overexpressed in E. coli and purified to near homogeneity; its molecular weight was 88,300, as expected. The K(m) values of the TPSP fusion enzyme for the sequential overall reaction from UDP-glucose and glucose 6-phosphate to trehalose were smaller than those of an equimolar mixture of TPS and TPP (TPS/TPP). However, the k(cat) values of TPSP were similar to those of TPS/TPP, resulting in a 3.5- to 4.0-fold increase in the catalytic efficiency (k(cat)/K(m)). The K(m) and k(cat) values of TPSP and TPP for the phosphatase reaction from T6P to trehalose were quite similar. This suggests that the increased catalytic efficiency results from the proximity of TPS and TPP in the TPSP fusion enzyme. The thermal stability of the TPSP fusion enzyme was quite similar to that of the TPS/TPP mixture, suggesting that the structure of each enzyme moiety in TPSP is unperturbed by intramolecular constraint. These results clearly demonstrate that the bifunctional fusion enzyme TPSP catalyzing sequential reactions has kinetic advantages over a mixture of both enzymes (TPS and TPP). These results are also supported by the in vivo accumulation of up to 0.48 mg of trehalose per g of cells after isopropyl-beta-D-thiogalactopyranoside treatment of cells harboring the construct encoding TPSP.
Figures





References
-
- Argos P. An investigation of oligopeptides linking domains in protein tertiary structures and possible candidates for general gene fusion. J Mol Biol. 1990;211:943–958. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Bülow L. Characterization of an artificial bifunctional enzyme, β-galactosidase/galactokinase, prepared by gene fusion. Eur J Biochem. 1987;163:443–448. - PubMed
-
- Bülow L, Mosbach K. Multienzyme systems obtained by gene fusion. Trends Biotechnol. 1991;9:226–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

