PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase
- PMID: 10831608
- PMCID: PMC2174826
- DOI: 10.1083/jcb.149.5.1039
PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase
Abstract
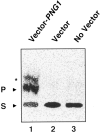
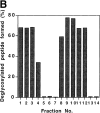
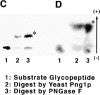
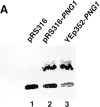
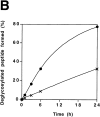
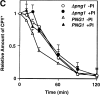
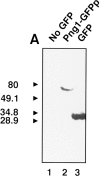
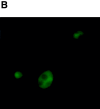
It has been proposed that cytoplasmic peptide:N-glycanase (PNGase) may be involved in the proteasome-dependent quality control machinery used to degrade newly synthesized glycoproteins that do not correctly fold in the ER. However, a lack of information about the structure of the enzyme has limited our ability to obtain insight into its precise biological function. A PNGase-defective mutant (png1-1) was identified by screening a collection of mutagenized strains for the absence of PNGase activity in cell extracts. The PNG1 gene was mapped to the left arm of chromosome XVI by genetic approaches and its open reading frame was identified. PNG1 encodes a soluble protein that, when expressed in Escherichia coli, exhibited PNGase activity. PNG1 may be required for efficient proteasome-mediated degradation of a misfolded glycoprotein. Subcellular localization studies indicate that Png1p is present in the nucleus as well as the cytosol. Sequencing of expressed sequence tag clones revealed that Png1p is highly conserved in a wide variety of eukaryotes including mammals, suggesting that the enzyme has an important function.
Figures




References
-
- Bebök Z., Mazzochi C., King S.A., Hong J.S., Sorscher E.J. The mechanism underlying cystic fibrosis transmembrane conductance regulator transport from the endoplasmic reticulum to the proteasome includes Sec61β and a cytosolic, deglycosylated intermediary. J. Biol. Chem. 1998;273:29873–29878. - PubMed
-
- Berger S., Menudier A., Julien R., Karamanos Y. Do de-N-glycosylation enzymes have an important role in plant cells? Biochimie. 1995;77:751–760. - PubMed
-
- Brodsky J.L., McCracken A.A. ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol. 1999;10:507–513. - PubMed
-
- Cresswell P., Hughes E.A. Protein degradationthe ins and outs of the matter. Curr. Biol. 1997;7:R552–R555. - PubMed
-
- de Virgilio M., Weninger H., Ivessa N.E. Ubiquitination is required for the retro-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J. Biol. Chem. 1998;273:9734–9743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

