The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading
- PMID: 10831617
- PMCID: PMC2174829
- DOI: 10.1083/jcb.149.5.1143
The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading
Abstract
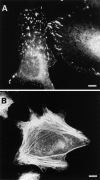
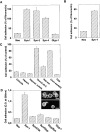
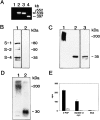
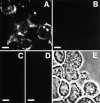
The ADAMs (a disintegrin and metalloprotease) family of proteins is involved in a variety of cellular interactions, including cell adhesion and ecto- domain shedding. Here we show that ADAM 12 binds to cell surface syndecans. Three forms of recombinant ADAM 12 were used in these experiments: the cys-teine-rich domain made in Escherichia coli (rADAM 12-cys), the disintegrin-like and cysteine-rich domain made in insect cells (rADAM 12-DC), and full-length human ADAM 12-S tagged with green fluorescent protein made in mammalian cells (rADAM 12-GFP). Mesenchymal cells specifically and in a dose-dependent manner attach to ADAM 12 via members of the syndecan family. After binding to syndecans, mesenchymal cells spread and form focal adhesions and actin stress fibers. Integrin beta1 was responsible for cell spreading because function-blocking monoclonal antibodies completely inhibited cell spreading, and chondroblasts lacking beta1 integrin attached but did not spread. These data suggest that mesenchymal cells use syndecans as the initial receptor for the ADAM 12 cysteine-rich domain-mediated cell adhesion, and then the beta1 integrin to induce cell spreading. Interestingly, carcinoma cells attached but did not spread on ADAM 12. However, spreading could be efficiently induced by the addition of either 1 mM Mn(2+) or the beta1 integrin-activating monoclonal antibody 12G10, suggesting that in these carcinoma cells, the ADAM 12-syndecan complex fails to modulate the function of beta1 integrin.
Figures





Comment in
-
Syndecan-regulated receptor signaling.J Cell Biol. 2000 May 29;149(5):995-8. doi: 10.1083/jcb.149.5.995. J Cell Biol. 2000. PMID: 10831602 Free PMC article. Review. No abstract available.
Similar articles
-
ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA.J Biol Chem. 2003 Mar 14;278(11):9576-84. doi: 10.1074/jbc.M208937200. Epub 2002 Dec 31. J Biol Chem. 2003. PMID: 12509413
-
Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells.Exp Cell Res. 2003 Jun 10;286(2):219-32. doi: 10.1016/s0014-4827(03)00126-5. Exp Cell Res. 2003. PMID: 12749851
-
Syndecan-1 signals independently of beta1 integrins during Raji cell spreading.Exp Cell Res. 2000 Sep 15;259(2):315-25. doi: 10.1006/excr.2000.4981. Exp Cell Res. 2000. PMID: 10964499
-
Syndecans and the lymphoid system.Leuk Lymphoma. 2000 Jul;38(3-4):271-81. doi: 10.3109/10428190009087018. Leuk Lymphoma. 2000. PMID: 10830734 Review.
-
Syndecans: multifunctional cell-surface co-receptors.Biochem J. 1997 Oct 1;327 ( Pt 1)(Pt 1):1-16. doi: 10.1042/bj3270001. Biochem J. 1997. PMID: 9355727 Free PMC article. Review.
Cited by
-
ADAM12 and alpha9beta1 integrin are instrumental in human myogenic cell differentiation.Mol Biol Cell. 2005 Feb;16(2):861-70. doi: 10.1091/mbc.e04-03-0226. Epub 2004 Dec 1. Mol Biol Cell. 2005. PMID: 15574885 Free PMC article.
-
The cysteine-rich domain regulates ADAM protease function in vivo.J Cell Biol. 2002 Dec 9;159(5):893-902. doi: 10.1083/jcb.200206023. Epub 2002 Dec 2. J Cell Biol. 2002. PMID: 12460986 Free PMC article.
-
The metalloproteinase ADAM-12 regulates bronchial epithelial cell proliferation and apoptosis.Cell Prolif. 2008 Dec;41(6):988-1001. doi: 10.1111/j.1365-2184.2008.00557.x. Cell Prolif. 2008. PMID: 19040574 Free PMC article.
-
Knockdown of a disintegrin A metalloprotease 12 (ADAM12) during adipogenesis reduces cell numbers, delays differentiation, and increases lipid accumulation in 3T3-L1 cells.Mol Biol Cell. 2018 Aug 1;29(15):1839-1855. doi: 10.1091/mbc.E17-07-0471. Epub 2018 May 30. Mol Biol Cell. 2018. PMID: 29846135 Free PMC article.
-
Proteoglycan signaling co-receptors: roles in cell adhesion, migration and invasion.Cell Signal. 2009 Nov;21(11):1548-58. doi: 10.1016/j.cellsig.2009.05.001. Epub 2009 May 8. Cell Signal. 2009. PMID: 19427900 Free PMC article. Review.
References
-
- Allen R.E., Dodson M.V., Luiten L.S. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp. Cell Res. 1984;152:154–160. - PubMed
-
- Almeida E.A., Huovila A.P., Sutherland A.E., Stephens L.E., Calarco P.G., Shaw L.M., Mercurio A.M., Sonnenberg A., Primakoff P., Myles D.G. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell. 1995;81:1095–1104. - PubMed
-
- Aszodi A., Pfeifer A., Ahmad M., Glauner M., Zhou X.H., Ny L., Andersson K.E., Kehrel B., Offermanns S., Fassler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:37–48. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

