Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury
- PMID: 10831618
- PMCID: PMC2174825
- DOI: 10.1083/jcb.149.5.1157
Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury
Abstract
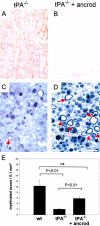
Tissue plasminogen activator (tPA) is a serine protease that converts plasminogen to plasmin and can trigger the degradation of extracellular matrix proteins. In the nervous system, under noninflammatory conditions, tPA contributes to excitotoxic neuronal death, probably through degradation of laminin. To evaluate the contribution of extracellular proteolysis in inflammatory neuronal degeneration, we performed sciatic nerve injury in mice. Proteolytic activity was increased in the nerve after injury, and this activity was primarily because of Schwann cell-produced tPA. To identify whether tPA release after nerve damage played a beneficial or deleterious role, we crushed the sciatic nerve of mice deficient for tPA. Axonal demyelination was exacerbated in the absence of tPA or plasminogen, indicating that tPA has a protective role in nerve injury, and that this protective effect is due to its proteolytic action on plasminogen. Axonal damage was correlated with increased fibrin(ogen) deposition, suggesting that this protein might play a role in neuronal injury. Consistent with this idea, the increased axonal degeneration phenotype in tPA- or plasminogen-deficient mice was ameliorated by genetic or pharmacological depletion of fibrinogen, identifying fibrin as the plasmin substrate in the nervous system under inflammatory axonal damage. This study shows that fibrin deposition exacerbates axonal injury, and that induction of an extracellular proteolytic cascade is a beneficial response of the tissue to remove fibrin. tPA/plasmin-mediated fibrinolysis may be a widespread protective mechanism in neuroinflammatory pathologies.
Figures





References
-
- Akassoglou K., Probert L., Kontogeorgos G., Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 1997;158:438–445. - PubMed
-
- Akassoglou K., Bauer J., Kassiotis G., Pasparakis M., Lassmann H., Kollias G., Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic micemodels for multiple sclerosis with primary oligodendrogliopathy. Am. J. Pathol. 1998;153:801–813. - PMC - PubMed
-
- Akenami F.O., Siren V., Wessman M., Koskiniemi M., Vaheri A. Tissue plasminogen activator gene expression in multiple sclerosis brain tissue. J. Neurol. Sci. 1999;165:71–76. - PubMed
-
- Arnold D.L. Magnetic resonance spectroscopyimaging axonal damage in MS. J. Neuroimmunol. 1999;98:2–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

