GeneClinics: a hybrid text/data electronic publishing model using XML applied to clinical genetic testing
- PMID: 10833163
- PMCID: PMC61429
- DOI: 10.1136/jamia.2000.0070267
GeneClinics: a hybrid text/data electronic publishing model using XML applied to clinical genetic testing
Abstract
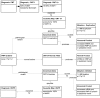
GeneClinics is an online genetic information resource consisting of descriptions of specific inherited disorders ("disease profiles") as well as information on the role of genetic testing in the diagnosis, management, and genetic counseling of patients with these inherited conditions. GeneClinics is intended to promote the use of genetic services in medical care and personal decision making by providing health care practitioners and patients with information on genetic testing for specific inherited disorders. GeneClinics is implemented as an object-oriented database containing a combination of data and semistructured text that is rendered as HTML for publishing a given "disease profile" on the Web. Content is acquired from authors via templates, converted to an XML document reflecting the underlying database schema (with tagging of embedded data), and then loaded into the database and subjected to peer review. The initial implementation of a production system and the first phase of population of the GeneClinics database content are complete. Further expansion of the content to cover more disease, significant scaling up of rate of content creation, and evaluation redesign are under way. The ultimate goal is to have an entry in GeneClinics for each entry in the GeneTests directory of medical genetics laboratories-that is, for each disease for which clinical genetic testing is available.
Figures





References
-
- Fink L, Collins F. The Human Genome Project: view from the National Institutes of Health. J Am Med Womens Assoc. 1997;52: 4-7. - PubMed
-
- Collins F. Medical and societal consequences of the Human Genome Project. N Engl J Med. 1999;341(1): 28-37. - PubMed
-
- Andrews L. Social, legal, and ethical implications of genetic testing. In: Fullarton J, Holtzman H, Motulsky AE (eds). Assessing Genetic Risks: Implications for Health and Social Policy. Washington, DC: National Academy Press, 1994. - PubMed
-
- Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility. J Clin Oncol. 1996;14: 1730-6. - PubMed
-
- Bird T, Bennett R. Why do DNA testing? Practical and ethical implications of new neurogenetics tests. Ann Neurol. 1995;38: 141-6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

