Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat
- PMID: 10835043
- PMCID: PMC2269951
- DOI: 10.1111/j.1469-7793.2000.t01-3-00405.x
Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat
Abstract
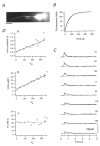
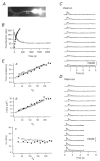
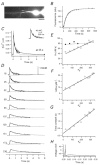
Endogenous calcium binding ratios (kappaS) in dendrites of cultured hippocampal neurons were estimated according to the single compartment model for transients in intracellular Ca2+ concentration ([Ca2+]). In addition, the electrophysiological characteristics of neurons were classified by their autaptic currents and intrinsic firing patterns. These data were analysed in order to determine whether a correlation between Ca2+ buffers and electrophysiological type exists. Ca2+ binding ratios of endogenous buffers were estimated by eliciting [Ca2+] transients with short depolarizations, while cells were loaded with fura-2. Two types of estimates could be obtained: one termed kappaS(tau), based on analysing time constants (tau) of [Ca2+] transients, and another termed kappaS(dCa), derived from an analysis of initial amplitudes of [Ca2+] transients. Values for kappaS(tau) and kappaS(dCa) were estimated as 57 +/- 10 (mean +/- s.d., n = 10) and 60 +/- 14 (n = 10), respectively, in excitatory neurons, and 130 +/- 50 (n = 11) and 150 +/- 70 (n = 11), respectively, in inhibitory neurons. The kappaS values of excitatory and inhibitory cells were significantly different from each other, regardless of the measurement method (Student's t test, P < 0.01). However, there was no significant difference in kappaS between the groups classified according to firing patterns. Although kappaS(tau) values were well matched to those of kappaS(dCa) in most excitatory cells, the two values did not agree in three out of the fourteen inhibitory cells investigated. In these cells, the first few [Ca2+] transients after obtaining the whole cell configuration displayed a double exponential decay, suggesting that buffers with slow binding kinetics, such as parvalbumin, are involved. This hypothesis is further explored in an accompanying paper.
Figures


References
-
- Baimbridge K, Celio M, Rogers J. Calcium-binding proteins in the nervous system. Trends in Neurosciences. 1992;15:303–308. - PubMed
-
- Baimbridge K, Miller J. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation, and olfactory bulb of the rat. Brain Research. 1982;245:223–229. - PubMed
-
- Celio MR. Parvalbumin in most γ-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231:995–997. - PubMed
-
- Connors B, Gutnick M. Intrinsic firing patterns of diverse neocortical neurons. Trends in Neurosciences. 1990;13:99–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

