Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat
- PMID: 10835051
- PMCID: PMC2269948
- DOI: 10.1111/j.1469-7793.2000.t01-1-00509.x
Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat
Abstract
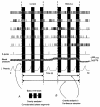
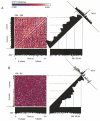
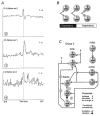
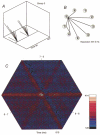
The regulation of gas exchange requires coordination of the respiratory and cardiovascular systems. Previous work suggested that medullary raphe neurones transform and transmit information from baroreceptors to neurones in the ventral respiratory group. This study tested the hypothesis that distributed brainstem neuronal assemblies are transiently reconfigured during the respiratory cycle and baroreceptor stimulation. Blood pressure was perturbed by intravenous injection of an alpha1-adrenergic receptor agonist, unilateral pressure changes in the carotid sinus, or occlusion of the descending aorta in 14 Dial-urethane anaesthetized, vagotomized, paralysed, artificially ventilated cats. Neurones were monitored simultaneously with microelectrode arrays in two or more of the following sites: n. raphe obscurus, n. raphe magnus, rostral and caudal ventrolateral medulla, and the nucleus tractus solitarii. Transient configurations of baroresponsive assemblies were detected with joint pericycle-triggered histograms, the gravitational representation, and related pattern detection methods. Data were also analysed with cycle-triggered histograms, peristimulus-time and cumulative sum histograms, cross-correlograms, spike-triggered averages of efferent phrenic activity, and joint impulse configuration scatter diagrams (snowflakes). Five to nine simultaneously recorded spike trains from control expiratory phases were compared with data from interleaved equal-duration time blocks from control inspiratory phases. In each of seven animals, significant impulse synchrony detected by gravity analysis was confined to one phase of the respiratory cycle. Repeated patterns of distributed synchrony confined to periods of altered baroreceptor activity were detected and involved neurones that individually did not change firing rates during stimulation. Snowflakes and logical cross-correlation analysis provided evidence for the cooperative actions of impulses in concurrently active parallel channels. In 12 of 17 pairs of neurones with at least one baroresponsive cell, joint pericycle-triggered histograms detected synchrony indicative of shared inputs or functional excitatory interactions that varied as a function of time in the respiratory cycle. Neurones in four of the pairs had no respiratory modulation of their individual firing rates. Data from eight other pairs were indicative of fluctuations in inhibition during the respiratory cycle. The results demonstrate repeated transient configurations of baroresponsive neuronal assemblies during the respiratory cycle, without concomitant firing rate changes in the constituent neurones, and suggest distributed network mechanisms for the modulation of baroreceptor-mediated reflexes.
Figures








References
-
- Abeles M. The quantification and graphic display of correlations among three spike trains. IEEE Transactions on Biomedical Engineering. 1983;30:235–239. - PubMed
-
- Adrian ED. The Mechanism of Nervous Action. Electrical Studies of the Neurone. London: Oxford University Press; 1932. p. 103.
-
- Aertsen AMHJ, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Research. 1985;340:341–354. - PubMed
-
- Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: Modulation of ‘effective connectivity’. Journal of Neurophysiology. 1989;61:900–917. - PubMed
-
- Arata A, Morris KF, Shannon R, Lindsey BG. Dynamic associations in distributed brainstem neural networks responsive to carotid baroreceptors. Neuroscience Research. 1991;16:S143.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

