RAP1 controls rhoptry targeting of RAP2 in the malaria parasite Plasmodium falciparum
- PMID: 10835342
- PMCID: PMC212767
- DOI: 10.1093/emboj/19.11.2435
RAP1 controls rhoptry targeting of RAP2 in the malaria parasite Plasmodium falciparum
Abstract
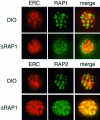
Rhoptry associated protein 1 (RAP1) and 2 (RAP2), together with a poorly described third protein RAP3, form the low molecular weight complex within the rhoptries of Plasmodium falciparum. These proteins are thought to play a role in erythrocyte invasion by the extracellular merozoite and are important vaccine candidates. We used gene-targeting technology in P.falciparum blood-stage parasites to disrupt the RAP1 gene, producing parasites that express severely truncated forms of RAP1. Immunoprecipitation experiments suggest that truncated RAP1 species did not complex with RAP2 and RAP3. Consistent with this were the distinct subcellular localizations of RAP1 and 2 in disrupted RAP1 parasites, where RAP2 does not traffic to the rhoptries but is instead located in a compartment that appears related to the lumen of the endoplasmic reticulum. These results suggest that RAP1 is required to localize RAP2 to the rhoptries, supporting the hypothesis that rhoptry biogenesis is dependent in part on the secretory pathway in the parasite. The observation that apparently host-protective merozoite antigens are not essential for efficient erythrocyte invasion has important implications for vaccine design.
Figures




References
-
- Andres D.A., Rhodes,J.D., Meisel,R.L. and Dixon,J.E. (1991) Characterisation of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J. Biol. Chem., 266, 14277–14288. - PubMed
-
- Bushell G.R., Ingram,L.T., Fardoulys,C.A. and Cooper,J.A. (1988) An antigenic complex in the rhoptries of Plasmodium falciparum. Mol. Biochem. Parasitol., 28, 105–112. - PubMed
-
- Camus D. and Hadley,T.J. (1985) A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science, 230, 553–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

