Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins
- PMID: 10835343
- PMCID: PMC212747
- DOI: 10.1093/emboj/19.11.2444
Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins
Abstract
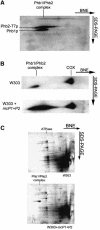
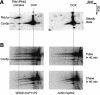
Prohibitins are ubiquitous, abundant and evolutionarily strongly conserved proteins that play a role in important cellular processes. Using blue native electrophoresis we have demonstrated that human prohibitin and Bap37 together form a large complex in the mitochondrial inner membrane. This complex is similar in size to the yeast complex formed by the homologues Phb1p and Phb2p. In yeast, levels of this complex are increased on co-overexpression of both Phb1p and Phb2p, suggesting that these two proteins are the only components of the complex. Pulse-chase experiments with mitochondria isolated from phb1/phb2-null and PHB1/2 overexpressing cells show that the Phb1/2 complex is able to stabilize newly synthesized mitochondrial translation products. This stabilization probably occurs through a direct interaction because association of mitochondrial translation products with the Phb1/2 complex could be demonstrated. The fact that Phb1/2 is a large multimeric complex, which provides protection of native peptides against proteolysis, suggests a functional homology with protein chaperones with respect to their ability to hold and prevent misfolding of newly synthesized proteins.
Figures


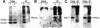

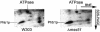
References
-
- Chen D.C., Yang,B.C. and Kuo,T.T. (1992) One-step transformation of yeast in stationary phase. Curr. Genet., 21, 83–84. - PubMed
-
- Coates P.J., Jamieson,D.J., Smart,K., Prescott,A.R. and Hall,P.A. (1997) The prohibitin family of mitochondrial proteins regulate replicative lifespan. Curr. Biol., 7, 607–610. - PubMed
-
- Decoster E., Simon,M., Hatat,D. and Faye,G. (1990) The MSS51 gene product is required for the translation of the COX1 mRNA in yeast mitochondria. Mol. Gen. Genet., 224, 111–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

