Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo
- PMID: 10835345
- PMCID: PMC212758
- DOI: 10.1093/emboj/19.11.2465
Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo
Abstract
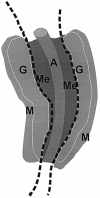
The aorta-gonad-mesonephros (AGM) region is a potent hematopoietic site within the mammalian embryo body, and the first place from which hematopoietic stem cells (HSCs) emerge. Within the complex embryonic vascular, excretory and reproductive tissues of the AGM region, the precise location of HSC development is unknown. To determine where HSCs develop, we subdissected the AGM into aorta and urogenital ridge segments and transplanted the cells into irradiated adult recipients. We demonstrate that HSCs first appear in the dorsal aorta area. Furthermore, we show that vitelline and umbilical arteries contain high frequencies of HSCs coincident with HSC appearance in the AGM. While later in development and after organ explant culture we find HSCs in the urogenital ridges, our results strongly suggest that the major arteries of the embryo are the most important sites from which definitive HSCs first emerge.
Figures



References
-
- Baldwin H.S. et al. (1994) Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development, 120, 2539–2553. - PubMed
-
- Bertwistle D., Walmsley,M.E., Read,E.M., Pizzey,J.A. and Patient,R.K. (1996) GATA factors and the origins of adult and embryonic blood in Xenopus: responses to retinoic acid. Mech. Dev., 57, 199–214. - PubMed
-
- Brown L., Rodaway,A., Schilling,T., Jowett,T., Ingham,P., Patient,R. and Sharrocks,A. (2000) Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor FLi-1 in wild-type and mutant zebrafish embryos. Mech. Dev., 90, 237–252. - PubMed
-
- Carmeliet P. et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature, 380, 435–439. - PubMed
-
- Chiquoine A. (1954) The identification, origin and migration of the primordial germ cells in the mouse embryo. Anat. Rec., 118, 135–146. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

