TREK-1 is a heat-activated background K(+) channel
- PMID: 10835347
- PMCID: PMC212769
- DOI: 10.1093/emboj/19.11.2483
TREK-1 is a heat-activated background K(+) channel
Abstract
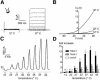
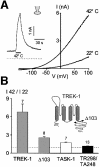
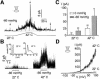
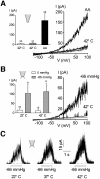
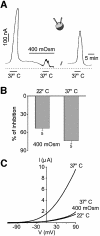
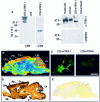
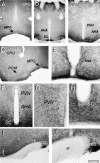
Peripheral and central thermoreceptors are involved in sensing ambient and body temperature, respectively. Specialized cold and warm receptors are present in dorsal root ganglion sensory fibres as well as in the anterior/preoptic hypothalamus. The two-pore domain mechano-gated K(+) channel TREK-1 is highly expressed within these areas. Moreover, TREK-1 is opened gradually and reversibly by heat. A 10 degrees C rise enhances TREK-1 current amplitude by approximately 7-fold. Prostaglandin E2 and cAMP, which are strong sensitizers of peripheral and central thermoreceptors, reverse the thermal opening of TREK-1 via protein kinase A-mediated phosphorylation of Ser333. Expression of TREK-1 in peripheral sensory neurons as well as in central hypothalamic neurons makes this K(+) channel an ideal candidate as a physiological thermoreceptor.
Figures
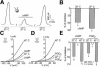

References
-
- Boulant J.A. (1998a) Cellular mechanisms of temperature sensitivity in hypothalamic neurons. Prog. Brain Res., 115, 3–8. - PubMed
-
- Boulant J.A. (1998b) Hypothalamic neurons. Mechanisms of sensitivity to temperature. Ann. N Y Acad. Sci., 856, 108–115. - PubMed
-
- Braun H.A., Bade,H. and Hensel,H. (1980) Static and dynamic discharge patterns of bursting cold fibers related to hypothetical receptor mechanisms. Pflugers Arch., 386, 1–9. - PubMed
-
- Caterina M.J. and Julius,D. (1999) Sense and specificity: a molecular identity for nociceptors. Curr. Opin. Neurobiol., 9, 525–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

