The level of DLDB/CHIP controls the activity of the LIM homeodomain protein apterous: evidence for a functional tetramer complex in vivo
- PMID: 10835358
- PMCID: PMC212760
- DOI: 10.1093/emboj/19.11.2602
The level of DLDB/CHIP controls the activity of the LIM homeodomain protein apterous: evidence for a functional tetramer complex in vivo
Abstract
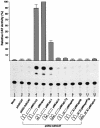
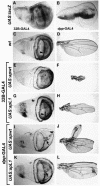
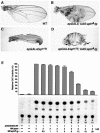
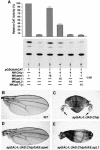
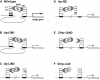
The LIM homeodomain (LIM-HD) protein Apterous (Ap) and its cofactor DLDB/CHIP control dorso- ventral (D/V) patterning and growth of Drosophila wing. To investigate the molecular mechanisms of Ap/CHIP function we altered their relative levels of expression and generated mutants in the LIM1, LIM2 and HD domains of Ap, as well as in the LIM-interacting and self-association domains of CHIP. Using in vitro and in vivo assays we found that: (i) the levels of CHIP relative to Ap control D/V patterning; (ii) the LIM1 and LIM2 domains differ in their contributions to Ap function; (iii) Ap HD mutations cause weak dominant negative effects; (iv) overexpression of ChipDeltaSAD mutants mimics Ap lack-of-function, and this dominant negative phenotype is caused by titration of Ap because it can be rescued by adding extra Ap; and (v) overexpression of ChipDeltaLID mutants also causes an Ap lack-of-function phenotype, but it cannot be rescued by extra Ap. These results support the model that the Ap-CHIP active complex in vivo is a tetramer.
Figures


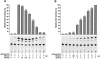

References
-
- Arber S. and Caroni,P. (1996) Specificity of single LIM motifs in targeting and LIM/LIM interactions in situ. Genes Dev., 10, 289–300. - PubMed
-
- Bach I. et al. (1999) RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nature Genet., 22, 394–399. - PubMed
-
- Blair S.S. (1993) Mechanisms of compartment formation: Evidence that non-proliferating cells do not play a role in defining the D/V lineage restriction in the developing wing of Drosophila. Development, 119, 339–351. - PubMed
-
- Brand A. and Perrimon,N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

