Tissue- and stage-specific modulation of Wingless signaling by the segment polarity gene lines
- PMID: 10837029
- PMCID: PMC316668
Tissue- and stage-specific modulation of Wingless signaling by the segment polarity gene lines
Abstract
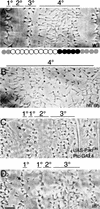
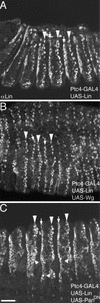
Wnt signaling controls a variety of developmental programs but the mechanisms by which the same signal leads to distinct outputs remain unclear. To address this question, we identified stage-specific modulators of Wingless (Wg) signaling in the Drosophila embryonic epidermis. We show that lines (lin) is essential for Wg-dependent patterning in dorsal epidermis. lin encodes a novel protein that acts cell-autonomously, downstream or in parallel to Armadillo (Arm) and upstream of Wg-dependent target genes. Lin can accumulate in nuclei of cells signaled by Wg, suggesting that signaling promotes entry of Lin into the nucleus, where it cooperates with Arm and Pangolin. Thus, a stage-specific modulator is used to mediate Wg signaling activity in dorsal patterning. Hedgehog (Hh) controls half of the parasegmental pattern dorsally and antagonizes Wg function to do so. Lin can accumulate in the cytoplasm of cells signaled by Hh, suggesting that Hh antagonizes Wg function by prohibiting Lin from entering the nucleus.
Figures







References
-
- Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J Biol Chem. 1999;274:35269–35277. - PubMed
-
- Alexandre C, Lecoutois M, Vincent J-P. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development. 1999;126:5689–5698. - PubMed
-
- ————— Embryonic and imaginal requirements for wg, a segment polarity gene in Drosophila. Dev Biol. 1988;125:96–108. - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
