In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers
- PMID: 10839805
- PMCID: PMC2213523
- DOI: 10.1084/jem.191.11.1895
In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers
Abstract
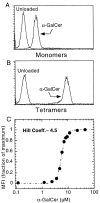
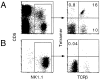
The CD1 family of major histocompatibility complex (MHC)-like molecules specializes in presenting lipid and glycolipid antigens to alpha/beta T lymphocytes, but little is known about the size of the CD1-restricted T cell population or the frequency of T lymphocytes specific for a given glycolipid antigen. Here, we report the generation and use of mouse CD1d1-glycolipid tetramers to visualize CD1d-restricted T cells. In contrast with previous BIAcore-based estimates of very short half-lives for CD1d-glycolipid complexes, we found that the dissociation rate of several different CD1d-glycolipid complexes was very slow. Fluorescent tetramers of mouse CD1d1 complexed with alpha-galactosylceramide (alphaGalCer), the antigen recognized by mouse Valpha14-Jalpha281/Vbeta8 and human Valpha24-JalphaQ/Vbeta11 natural killer T (NKT) cell T cell receptors (TCRs), allowed us for the first time to accurately describe, based on TCR specificity, the entire population of NKT cells in vivo and to identify a previously unrecognized population of NK1.1-negative "NKT" cells, which expressed a different pattern of integrins. In contrast, natural killer (NK) cells failed to bind the tetramers either empty or loaded with alphaGalCer, suggesting the absence of a CD1d-specific, antigen-nonspecific NK receptor. Mouse CD1d1-alphaGalCer tetramers also stained human NKT cells, indicating that they will be useful for probing a range of mouse and human conditions such as insulin-dependent diabetes mellitus, tumor rejection, and infectious diseases where NKT cells play an important role.
Figures




References
-
- Calabi F., Jarvis J.M., Martin L.H., Milstein C. Two classes of CD1 genes. Eur. J. Immunol. 1989;19:285–292. - PubMed
-
- Porcelli S.A., Modlin R.L. The CD1 systemantigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. - PubMed
-
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

