Enhanced growth of primary tumors in cancer-prone mice after immunization against the mutant region of an inherited oncoprotein
- PMID: 10839809
- PMCID: PMC2213520
- DOI: 10.1084/jem.191.11.1945
Enhanced growth of primary tumors in cancer-prone mice after immunization against the mutant region of an inherited oncoprotein
Abstract
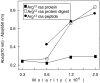
One major objective of tumor immunologists is to prevent cancer development in individuals at high risk. (TG.AC x C57BL/6)F1 mice serve as a model for testing the feasibility of this objective. The mice carry in the germline a mutant ras oncogene that has an arginine at codon 12 instead of glycine present in the wild-type, and after physical (wounding) or chemical promotion, these mice have a high probability for developing papillomas that progress to cancer. Furthermore, F1 mice immunized with Arg(12) mutant ras peptide in complete Freund's adjuvant (CFA) develop T cells within 10 d that proliferate in vitro on stimulation with the Arg(12) mutant ras peptide. Within 14 d, these mice have delayed-type hypersensitivity to the peptide. Immunization with CFA alone or with a different Arg(12) mutant ras peptide in CFA induced neither response. To determine the effect of immunization on development of tumors, mice immunized 3 wk earlier were painted on the back with phorbol 12-myristate 13-acetate every 3 d for 8 wk. The time of appearance and the number of papillomas were about the same in immunized and control mice, but the tumors grew faster and became much larger in the mice immunized with the Arg(12) mutant ras peptide. Thus, the immunization failed to protect against growth of papillomas. The peptide-induced CD4(+) T cells preferentially recognized the peptide but not the native mutant ras protein. On the other hand, mice immunized with Arg(12) mutant ras peptide and bearing papillomas had serum antibodies that did bind native mutant ras protein. Together, these studies indicate that active immunization of cancer-prone individuals may result in immune responses that fail to eradicate mutant oncogene-expressing tumor cells, but rather induce a remarkable enhancement of tumor growth.
Figures

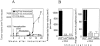
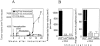







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

