A functional-phylogenetic classification system for transmembrane solute transporters
- PMID: 10839820
- PMCID: PMC98997
- DOI: 10.1128/MMBR.64.2.354-411.2000
A functional-phylogenetic classification system for transmembrane solute transporters
Abstract
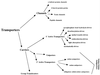
A comprehensive classification system for transmembrane molecular transporters has been developed and recently approved by the transport panel of the nomenclature committee of the International Union of Biochemistry and Molecular Biology. This system is based on (i) transporter class and subclass (mode of transport and energy coupling mechanism), (ii) protein phylogenetic family and subfamily, and (iii) substrate specificity. Almost all of the more than 250 identified families of transporters include members that function exclusively in transport. Channels (115 families), secondary active transporters (uniporters, symporters, and antiporters) (78 families), primary active transporters (23 families), group translocators (6 families), and transport proteins of ill-defined function or of unknown mechanism (51 families) constitute distinct categories. Transport mode and energy coupling prove to be relatively immutable characteristics and therefore provide primary bases for classification. Phylogenetic grouping reflects structure, function, mechanism, and often substrate specificity and therefore provides a reliable secondary basis for classification. Substrate specificity and polarity of transport prove to be more readily altered during evolutionary history and therefore provide a tertiary basis for classification. With very few exceptions, a phylogenetic family of transporters includes members that function by a single transport mode and energy coupling mechanism, although a variety of substrates may be transported, sometimes with either inwardly or outwardly directed polarity. In this review, I provide cross-referencing of well-characterized constituent transporters according to (i) transport mode, (ii) energy coupling mechanism, (iii) phylogenetic grouping, and (iv) substrates transported. The structural features and distribution of recognized family members throughout the living world are also evaluated. The tabulations should facilitate familial and functional assignments of newly sequenced transport proteins that will result from future genome sequencing projects.
Figures
References
-
- Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Ajouz B, Berrier C, Garrigues A, Besnard M, Ghazi A. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J Biol Chem. 1998;273:26670–26674. - PubMed
-
- Axelsen K B, Palmgren M G. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

