Type I restriction systems: sophisticated molecular machines (a legacy of Bertani and Weigle)
- PMID: 10839821
- PMCID: PMC98998
- DOI: 10.1128/MMBR.64.2.412-434.2000
Type I restriction systems: sophisticated molecular machines (a legacy of Bertani and Weigle)
Abstract
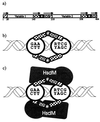
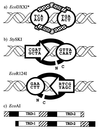
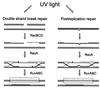
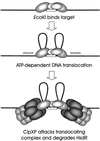
Restriction enzymes are well known as reagents widely used by molecular biologists for genetic manipulation and analysis, but these reagents represent only one class (type II) of a wider range of enzymes that recognize specific nucleotide sequences in DNA molecules and detect the provenance of the DNA on the basis of specific modifications to their target sequence. Type I restriction and modification (R-M) systems are complex; a single multifunctional enzyme can respond to the modification state of its target sequence with the alternative activities of modification or restriction. In the absence of DNA modification, a type I R-M enzyme behaves like a molecular motor, translocating vast stretches of DNA towards itself before eventually breaking the DNA molecule. These sophisticated enzymes are the focus of this review, which will emphasize those aspects that give insights into more general problems of molecular and microbial biology. Current molecular experiments explore target recognition, intramolecular communication, and enzyme activities, including DNA translocation. Type I R-M systems are notable for their ability to evolve new specificities, even in laboratory cultures. This observation raises the important question of how bacteria protect their chromosomes from destruction by newly acquired restriction specifities. Recent experiments demonstrate proteolytic mechanisms by which cells avoid DNA breakage by a type I R-M system whenever their chromosomal DNA acquires unmodified target sequences. Finally, the review will reflect the present impact of genomic sequences on a field that has previously derived information almost exclusively from the analysis of bacteria commonly studied in the laboratory.
Figures



References
-
- Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. - PubMed
-
- Althorpe N J, Chilley P M, Thomas A T, Brammar W J, Wilkins B M. Transient transcriptional activation of the Incl1 plasmid anti-restriction gene (ardA) and SOS inhibition gene (psiB) early in conjugating recipient bacteria. Mol Microbiol. 1999;31:133–142. - PubMed
-
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

