The PHSRN sequence induces extracellular matrix invasion and accelerates wound healing in obese diabetic mice
- PMID: 10841512
- PMCID: PMC300849
- DOI: 10.1172/JCI8527
The PHSRN sequence induces extracellular matrix invasion and accelerates wound healing in obese diabetic mice
Abstract
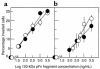
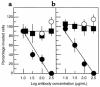
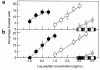
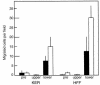
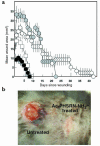
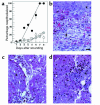
The PHSRN sequence of the plasma fibronectin (pFn) cell-binding domain induces human keratinocytes and fibroblasts to invade the naturally serum-free extracellular matrices of sea urchin embryos. The potency of acetylated, amidated PHSRN (Ac-PHSRN-NH(2)) is significantly increased, making it more active on a molar basis than the 120-kDa cell-binding domain of pFn. Arginine is important to this activity because PHSAN and PHSEN are inactive, as is a randomized sequence peptide, Ac-HSPNR-NH(2). One treatment with Ac-PHSRN-NH(2) stimulates reepithelialization and contraction of dermal wounds in healing-impaired, obese diabetic C57BL6/KsJ db/db mice. Wound closure is equally rapid in treated db/db and db/+ mice and may be more rapid than in untreated nondiabetic db/+ littermates. In contrast, treatment with either Ac-HSPNR-NH(2) or normal saline (NS) has no effect. Analysis of sectioned db/db wounds shows that, in contrast to treatment with Ac-HSPNR-NH(2) or NS, a single Ac-PHSRN-NH(2) treatment stimulates keratinocyte and fibroblast migration into wounds, enhances fibroplasia and vascularization in the provisional matrix, and stimulates the formation of prominent fibers that may be associated with wound contraction.
Figures


Comment in
-
Fibronectin peptides in cell migration and wound repair.J Clin Invest. 2000 Jun;105(11):1507-9. doi: 10.1172/JCI10119. J Clin Invest. 2000. PMID: 10841505 Free PMC article. No abstract available.
References
-
- Clark, R.A.F. 1996. Wound repair: overview and general considerations. In The molecular and cellular biology of wound repair. 2nd edition. R.F. Clark, editor. Plenum Press. New York, New York, USA. 3–50.
-
- Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Mosher DF. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. - PubMed
-
- Clark RAF, et al. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982;70:264–269. - PubMed
-
- Clark RAF, Quinn JH, Winn HJ, Colvin RB. Fibronectin beneath reepithelializing epidermis in vivo: sources and significance. J Invest Dermatol. 1983;80(Suppl.):26S–30S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

