Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate
- PMID: 10841579
- PMCID: PMC18759
- DOI: 10.1073/pnas.97.12.6838
Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate
Abstract
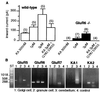
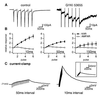
We report the presence of kainate receptors (KARs) in cerebellar Golgi cells of wild-type but not GluR6-deficient mice. Parallel fiber stimulation activates KAR-mediated synaptic currents [KAR-excitatory postsynaptic currents (EPSCs)] of small amplitude. KAR-EPSCs greatly differ from synaptic currents mediated by alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors (AMPAR-EPSCs) at the same synapse. KAR-EPSCs display slow rise and decay time and summate in response to a train of stimulations. By using PDA, a low-affinity competitive antagonist and agents that modify the clearance of glutamate, we show that these properties cannot be explained by diffusion of glutamate outside of the synaptic cleft and activation of extrasynaptic KARs. These data suggest that the slow kinetic of KAR-EPSCs is due to intrinsic properties of KARs being localized at postsynaptic sites. The contrasting properties of KAR- and AMPAR-EPSCs in terms of kinetics and summation offer the possibility for a glutamatergic synapse to integrate excitatory inputs over two different time scales.
Figures




References
-
- Bettler B, Mulle C. Neuropharmacology. 1995;34:123–139. - PubMed
-
- Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. - PubMed
-
- Chittajallu R, Braithwaite S, Clarke V, Henley J. Trends Pharmacol Sci. 1999;20:26–35. - PubMed
-
- Clarke V R, Ballyk B A, Hoo K H, Mandelzys A, Pellizzari A, Bath C P, Thomas J, Sharpe E F, Davies C H, Ornstein P L, et al. Nature (London) 1997;389:599–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

