Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse
- PMID: 10844011
- PMCID: PMC6772456
- DOI: 10.1523/JNEUROSCI.20-12-04423.2000
Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse
Abstract
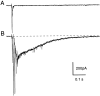
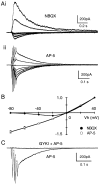
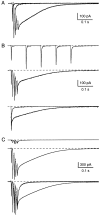
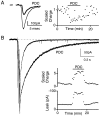
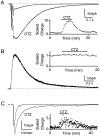
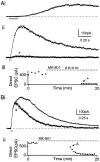
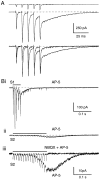
Although neurons often fire in bursts, most of what is known about glutamate signaling and postsynaptic receptor activation is based on experiments using single stimuli. Here we examine the activation of ionotropic glutamate receptors by bursts at the parallel fiber to stellate cell synapse. We show that brief stimulus trains generate prolonged AMPA receptor (AMPAR)- and NMDA receptor (NMDAR)-mediated EPSCs recorded in whole-cell voltage clamp. These EPSCs contrast with the rapid AMPAR-mediated EPSC evoked by a single stimulus. The prolonged AMPAR-mediated EPSC is promoted by high-frequency and high-intensity trains and can persist for hundreds of milliseconds. This EPSC is also increased by l-trans-2,4-PDC, an inhibitor of glutamate transporters, suggesting that these transporters usually limit the synaptic response to trains. These prolonged EPSCs reflect both receptor properties and a long-lasting glutamate signal. In addition, several experiments demonstrate that glutamate spillover can contribute to receptor activation. First, imaging stimulus-evoked changes in presynaptic calcium establishes that distinct parallel fiber bands can be activated. Second, activation of parallel fibers that do not directly synapse onto a given stellate cell can evoke indirect AMPAR- and NMDAR-mediated EPSCs in that cell. Third, experiments using the use-dependent NMDAR blocker MK-801 show that these indirect EPSCs reflect glutamate spillover in response to trains. Together, these findings indicate that stimulus trains can generate a sustained and widespread glutamate signal that can in turn evoke large and prolonged EPSCs mediated by ionotropic glutamate receptors. These synaptic properties may have important functional consequences for stellate cell firing.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
