Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals
- PMID: 10844015
- PMCID: PMC6772452
- DOI: 10.1523/JNEUROSCI.20-12-04462.2000
Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals
Abstract
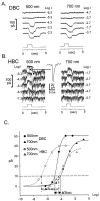
We correlated the morphology of salamander bipolar cells with characteristics of their light responses, recorded under voltage-clamp conditions. Twelve types of bipolar cells were identified, each displaying a unique morphology and level(s) of axon terminal stratification in the inner plexiform layer (IPL) and exhibiting light responses that differed with respect to polarity, kinetics, the relative strengths of rod and cone inputs, and characteristics of spontaneous EPSCs (sEPSCs) and IPSCs. In addition to the well known segregation of visual information into ON and OFF channels along the depth of the IPL, we found an overlying mapping of spectral information in this same dimension, with cone signals being transmitted predominantly to the central IPL and rod signals being sent predominantly to the margins of the IPL. The kinetics of bipolar cell responses correlated with this segregation of ON and OFF and of rod and cone information in the IPL. At light offset, rod-dominated cells displayed larger slow cationic current tails and smaller rapid overshoot responses than did cone-dominated cells. sEPSCs were generally absent in depolarizing bipolar cells but present in all hyperpolarizing bipolar cells (HBCs) and larger in rod-dominated HBCs than in cone-dominated HBCs. Inhibitory chloride currents, elicited both at light onset and light offset, tended to be larger for cone-dominated cells than for rod-dominated cells. This orderly segregation of visual signals along the depth of the IPL simplifies the integration of visual information in the retina, and it begins a chain of parallel processing in the visual system.
Figures


References
-
- Ammermuller J, Kolb H. The organization of the turtle inner retina. I. ON- and OFF-center pathways. J Comp Neurol. 1995;358:1–34. - PubMed
-
- Ashmore JF, Copenhagen DR. Different postsynaptic events in two types of retinal bipolar cell. Nature. 1980;288:84–86. - PubMed
-
- Ashmore JF, Falk G. Photon-like signals following weak rhodopsin bleaches. Nature. 1981;289:489–491. - PubMed
-
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philos Trans R Soc Lond B Biol Sci. 1969;255:109–194. - PubMed
-
- Boycott BB, Wassle H. Morphological classification of bipolar cells in the macaque monkey retina. Eur J Neurosci. 1991;361:1069–1088. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
