Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules
- PMID: 10844024
- PMCID: PMC6772458
- DOI: 10.1523/JNEUROSCI.20-12-04545.2000
Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules
Abstract
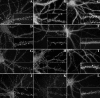
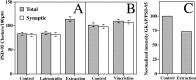
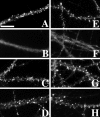
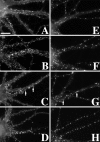
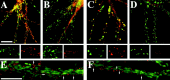
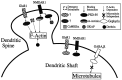
The mechanisms responsible for anchoring molecular components of postsynaptic specializations in the mammalian brain are not well understood but are presumed to involve associations with cytoskeletal elements. Here we build on previous studies of neurotransmitter receptors (Allison et al., 1998) to analyze the modes of attachment of scaffolding and signal transducing proteins of both glutamate and GABA postsynaptic sites to either the microtubule or microfilament cytoskeleton. Hippocampal pyramidal neurons in culture were treated with latrunculin A to depolymerize actin, with vincristine to depolymerize microtubules, or with Triton X-100 to extract soluble proteins. The synaptic clustering of PSD-95, a putative NMDA receptor anchoring protein and a core component of the postsynaptic density (PSD), was unaffected by actin depolymerization, microtubule depolymerization, or detergent extraction. The same was largely true for GKAP, a PSD-95-interacting protein. In contrast, the synaptic clustering of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII)alpha, another core component of the PSD, was completely dependent on an intact actin cytoskeleton and was partially disrupted by detergent. Drebrin and alpha-actinin-2, actin-binding proteins concentrated in spines, were also dependent on F-actin for synaptic localization but were unaffected by detergent extraction. Surprisingly, the subcellular distributions of the inhibitory synaptic proteins GABA(A)R and gephyrin, which has a tubulin-binding motif, were unaffected by depolymerization of microtubules or actin or by detergent extraction. These studies reveal an unsuspected heterogeneity in the modes of attachment of postsynaptic proteins to the cytoskeleton and support the idea that PSD-95 and gephyrin may be core scaffolding components independent of the actin or tubulin cytoskeleton.
Figures


References
-
- Adam G, Matus A. Role of actin in the organisation of brain postsynaptic densities. Brain Res Mol Brain Res. 1996;43:246–250. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–442. - PubMed
-
- Bensch KG, Marantz R, Wisniewski H, Shelanski M. Induction in vitro of microtubular crystals by vinca alkaloids. Science. 1969;165:495–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
