Lens injury stimulates axon regeneration in the mature rat optic nerve
- PMID: 10844031
- PMCID: PMC6772462
- DOI: 10.1523/JNEUROSCI.20-12-04615.2000
Lens injury stimulates axon regeneration in the mature rat optic nerve
Abstract
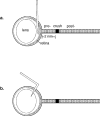
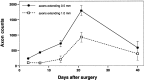
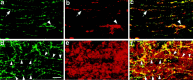
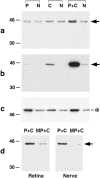
In mature mammals, retinal ganglion cells (RGCs) are unable to regenerate their axons after optic nerve injury, and they soon undergo apoptotic cell death. However, a small puncture wound to the lens enhances RGC survival and enables these cells to regenerate their axons into the normally inhibitory environment of the optic nerve. Even when the optic nerve is intact, lens injury stimulates macrophage infiltration into the eye, Müller cell activation, and increased GAP-43 expression in ganglion cells across the entire retina. In contrast, axotomy, either alone or combined with intraocular injections that do not infringe on the lens, causes only a minimal change in GAP-43 expression in RGCs and a minimal activation of the other cell types. Combining nerve injury with lens puncture leads to an eightfold increase in RGC survival and a 100-fold increase in the number of axons regenerating beyond the crush site. Macrophage activation appears to play a key role, because intraocular injections of Zymosan, a yeast cell wall preparation, stimulated monocytes in the absence of lens injury and induced RGCs to regenerate their axons into the distal optic nerve.
Figures







References
-
- Aguayo AJ, Vidal-Sanz M, Villegas-Perez MP, Bray GM. Growth and connectivity of axotomized retinal neurons in adult rats with optic nerves substituted by PNS grafts linking the eye and the midbrain. Ann NY Acad Sci. 1987;495:1–9. - PubMed
-
- Battisti WP, Wang J, Bozek K, Murray M. Macrophages, microglia, and astrocytes are rapidly activated after crush injury of the goldfish optic nerve: a light and electron microscopic analysis. J Comp Neurol. 1995;354:306–320. - PubMed
-
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
