Enhanced apoptosis of T cells in common variable immunodeficiency (CVID): role of defective CD28 co-stimulation
- PMID: 10844530
- PMCID: PMC1905559
- DOI: 10.1046/j.1365-2249.2000.01239.x
Enhanced apoptosis of T cells in common variable immunodeficiency (CVID): role of defective CD28 co-stimulation
Abstract
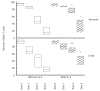
CVID is a primary immune disorder in which hypogammaglobulinaemia may be associated with a number of T cell defects including lymphopenia, anergy, impaired lymphocyte proliferation and deficient cytokine secretion. In this study we show that T cells of CVID subjects, in comparison with control T cells, undergo spontaneous apoptosis in culture and markedly accelerated apoptosis after gamma-irradiation. Although costimulation of the CD28 receptor following engagement of the TCR/CD3 receptor normally provides a second signal necessary for IL-2 secretion, CD28 costimulation in CVID does not significantly increase IL-2 production, nor does this combination of activators enhance the survival of irradiated CVID T cells, as it does for cultured normal T cells. Addition of IL-2 enhances CVID T cell survival, suggesting that the IL-2 signalling pathways are normal. CVID T cells have similar expression of Bcl-2 to control T cells. CD3 stimulation up-regulates T cell expression of bcl-xL mRNA for normal T cells, but anti-CD28 does not augment bcl-xL expression for CVID subjects with accelerated apoptosis. Defects of the CD28 receptor pathway, leading to cytokine deprivation and dysregulation of bcl-xL, could lead to poor T cell viability and some of the cellular defects observed in CVID.
Figures




References
-
- Report of a WHO Scientific Group. Clin Exp Immunol. 1997;109(Suppl.1):1–28. - PubMed
-
- Spickett GP, Farrant J, North ME, Zhang J, Morgan L, Webster Adb. Common variable immunodeficiency: how many diseases? Immunol Today. 1997;18:325–8. - PubMed
-
- Sneller MC. Sneller MC, editor. Clinical spectrum of common variable immunodeficiency. New insights into common variable immunodeficiency. Ann Intern Med. 1993;118:720–30. Moderator. - PubMed
-
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunologic features of 248 patients. Clin Immunol. 1999;92:34–48. - PubMed
-
- Hermaszewski RA, Webster Adb. Primary hypogammaglobulinemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

