The neurofibromatosis type 1 (Nf1) tumor suppressor is a modifier of carcinogen-induced pigmentation and papilloma formation in C57BL/6 mice
- PMID: 10844550
- PMCID: PMC2862652
- DOI: 10.1046/j.1523-1747.2000.00994.x
The neurofibromatosis type 1 (Nf1) tumor suppressor is a modifier of carcinogen-induced pigmentation and papilloma formation in C57BL/6 mice
Abstract
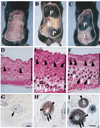
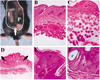
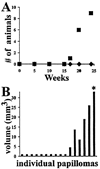
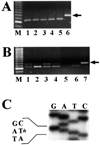
There is increasing evidence implicating the human NF1 gene in epithelial carcinogenesis. To test if NF1 can play a part in skin tumor formation, we analyzed effects of the skin cancer initiator dimethylbenz-anthracene and/or the tumor promoter 12-O-tetradecanoyl-13-acetylphorbol on mice heterozygous for null mutations in Nf1 (Nf1+/-). Mice were on the C57BL/6 background, noted for resistance to chemical carcinogens. Nf1+/- mice (18 of 24) developed papillomas after treatment with dimethylbenzanthracene and 12-O-tetradecanoyl-13-acetylphorbol; papillomas did not develop in wild-type C57BL/6 mice nor Nf1+/- mice treated with 12-O-tetradecanoyl-13-acetylphorbol alone. All papillomas analyzed (six of six) had mutations in codon 61 of H-ras, demonstrating strong cooperation between the Nf1 GTPase activating protein for Ras, neurofibromin, and Ras-GTP. After exposure to 12-O-tetradecanoyl-13-acetylphorbol, Nf1+/- keratinocytes showed significant, sustained, increases in proliferation, implicating Nf1 in phorbol ester responsive pathways. Thus, Nf1 levels regulate the response of keratinocytes to 12-O-tetradecanoyl-13-acetylphorbol. Nf1+/- mice also showed a 2-fold increase in the development of pigmented skin patches stimulated by dimethylbenzanthracene; patches were characterized by hair follicles in anagen phase, implicating keratinocytes in the aberrant hyperpigmentation. Our results show that mutation in the Nf1 gene causes abnormal keratinocyte proliferation that can be revealed by environmental assaults such as carcinogen exposure. The data support a plausible role for NF1 mutation in human epithelial carcinogenesis.
Figures

References
-
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumor cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. - PubMed
-
- Binder RL, Gallagher PM, Johnson GR, Stockman SL, Smith BJ, Sundberg JP, Conti CJ. Evidence that initiated keratinocytes clonally expand into multiple existing hair follicles during papilloma histogenesis in SENCAR mouse skin. Mol Carcinogenesis. 1997;20:151–158. - PubMed
-
- Bollag G, Clapp D, Shih S, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nature Genet. 1996;12:144–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

