Suppression of acute viremia by short-term postexposure prophylaxis of simian/human immunodeficiency virus SHIV-RT-infected monkeys with a novel reverse transcriptase inhibitor (GW420867) allows for development of potent antiviral immune responses resulting in efficient containment of infection
- PMID: 10846052
- PMCID: PMC112067
- DOI: 10.1128/jvi.74.13.5747-5753.2000
Suppression of acute viremia by short-term postexposure prophylaxis of simian/human immunodeficiency virus SHIV-RT-infected monkeys with a novel reverse transcriptase inhibitor (GW420867) allows for development of potent antiviral immune responses resulting in efficient containment of infection
Abstract
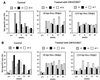
A nonnucleoside reverse transcriptase (RT) inhibitor, GW420867, was tested for postexposure prophylaxis (PEP) in rhesus macaques experimentally infected with 100 50% tissue culture infective doses of a chimeric simian/human immunodeficiency virus (SHIV) containing the RT gene of HIV-1 (SHIV-RT). Animals were either mock treated, or treated for 4 weeks starting at 8 or 24 h postinfection (p.i.) with GW420867. While such therapy led to undetectable plasma viremia in three of six monkeys, a transient plasma viremia was noted in the other three treated animals at 2 to 4 weeks following cessation of therapy. Following this transient viremia all drug-treated animals showed low or undetectable levels of plasma viremia up to the last sample examined at 90 weeks p.i. Despite low and/or undetectable viremia, virus-specific cytotoxic T lymphocyte and viral Env-specific proliferative responses were seen in the peripheral blood mononuclear cells of both mock- and drug-treated animals as early as 3 weeks p.i. Such virus-specific cellular responses, however, were better maintained in the drug-treated animals than the mock-treated animals. In contrast to the virus-specific cellular response, the magnitude and kinetics of virus specific humoral responses appeared to correlate with the detection of viremia. These data support the view that a short-term PEP with GW420867 permits the generation and maintenance of long-lasting virus-specific cell-mediated immune responses while markedly reducing viral loads to undetectable levels for a prolonged period of time (90 weeks) and leads to long-term disease protection. This model provides a unique means to define mechanisms and correlates of disease protection.
Figures


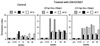
References
-
- Akari H, Mori K, Terao K, Otani I, Fukasawa M, Mukai R, Yoshikawa Y. In vitro immortalization of Old World monkey T lymphocytes with Herpesvirus Saimiri. Virology. 1996;218:382–388. - PubMed
-
- Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
-
- Balter M. Can immune systems be trained to fight HIV? Science. 1999;286:1470–1471. - PubMed
-
- Cocchi F, Devico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. - PubMed
-
- Daniel M D, Kirchhoff K, Czajak S C, Shegal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

