Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer
- PMID: 10846088
- PMCID: PMC112103
- DOI: 10.1128/jvi.74.13.6050-6057.2000
Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer
Abstract
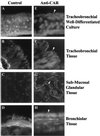
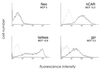
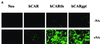
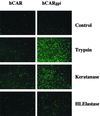
Lumenal delivery of adenovirus vectors (AdV) results in inefficient gene transfer to human airway epithelium. The human coxsackievirus and adenovirus receptor (hCAR) was detected by immunofluorescence selectively at the basolateral surfaces of freshly excised human airway epithelial cells, suggesting that the absence of apical hCAR constitutes a barrier to adenovirus-mediated gene delivery in vivo. In transfected polarized Madin-Darby canine kidney cells, wild-type hCAR was expressed selectively at the basolateral membrane, whereas hCAR lacking the transmembrane and/or cytoplasmic domains was expressed on both the basolateral and apical membranes. Cells expressing apical hCAR still were not efficiently transduced by AdV applied to the apical surface. However, after the cells were treated with agents that remove components of the apical surface glycocalyx, AdV transduction occurred. These results indicate that adenovirus can infect via receptors located at the apical cell membrane but that the glycocalyx impedes interaction of AdV with apical receptors.
Figures





References
-
- Arcasoy S M, Latoche J, Gondor M, Watkins S C, Henderson R A, Hughey R, Finn O J, Pilewski J M. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. - PubMed
-
- Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Bhaskar K, O'Sullivan D, Opaskar-Hincman H, Reid L, Coles S. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10:401–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

