Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans
- PMID: 10846094
- PMCID: PMC112109
- DOI: 10.1128/jvi.74.13.6105-6116.2000
Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans
Abstract
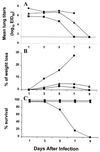
Previously, we observed that several virulent influenza A (H5N1) viruses which caused severe or fatal disease in humans were also lethal in BALB/c mice following dissemination of the virus to solid organs, including the brain. In contrast, one particular human H5N1 virus was nonlethal in mice and showed no evidence of systemic spread. To compare H5N1 viruses of varying pathogenicity for their ability to alter the mammalian immune system, mice were infected with either influenza A/Hong Kong/483/97 (HK/483) (lethal) or A/Hong Kong/486/97 (HK/486) (nonlethal) virus and monitored for lymphocyte depletion in the blood, lungs, and lymphoid tissue. Intranasal infection with HK/483 resulted in a significant decrease in the total number of circulating leukocytes evident as early as day 2 postinfection. Differential blood counts demonstrated up to an 80% drop in lymphocytes by day 4 postinfection. In contrast, nonlethal HK/486-infected mice displayed only a transient drop of lymphocytes during the infectious period. Analysis of lung and lymphoid tissue from HK/483-infected mice demonstrated a reduction in the number of CD4(+) and CD8(+) T cells and reduced synthesis of the cytokines interleukin-1beta and gamma interferon and the chemokine macrophage inflammatory protein compared with HK/486-infected mice. In contrast, the cytokine and chemokine levels were increased in the brains of mice infected with HK/483 but not HK/486. Evidence of apoptosis in the spleen and lung of HK/483-infected mice was detected in situ, suggesting a mechanism for lymphocyte destruction. These results suggest that destructive effects on the immune system may be one factor that contributes to the pathogenesis of H5N1 viruses in mammalian hosts.
Figures





Similar articles
-
Distinct pathogenesis of hong kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses.J Virol. 2000 Feb;74(3):1443-50. doi: 10.1128/jvi.74.3.1443-1450.2000. J Virol. 2000. PMID: 10627555 Free PMC article.
-
Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans.J Virol. 2000 Jul;74(14):6592-9. doi: 10.1128/jvi.74.14.6592-6599.2000. J Virol. 2000. PMID: 10864673 Free PMC article.
-
Pathogenesis of Hong Kong H5N1 influenza virus NS gene reassortants in mice: the role of cytokines and B- and T-cell responses.J Gen Virol. 2005 Apr;86(Pt 4):1121-1130. doi: 10.1099/vir.0.80663-0. J Gen Virol. 2005. PMID: 15784906
-
[Cytokine storm in avian influenza].Mikrobiyol Bul. 2008 Apr;42(2):365-80. Mikrobiyol Bul. 2008. PMID: 18697437 Review. Turkish.
-
Immunity to avian influenza A viruses.Rev Sci Tech. 2009 Apr;28(1):175-85. doi: 10.20506/rst.28.1.1857. Rev Sci Tech. 2009. PMID: 19618625 Review.
Cited by
-
Potential role of HPA axis and sympathetic nervous responses in depletion of B cells induced by H9N2 avian influenza virus infection.PLoS One. 2012;7(12):e51029. doi: 10.1371/journal.pone.0051029. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251416 Free PMC article.
-
Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11531-6. doi: 10.1073/pnas.0914994107. Epub 2010 Jun 7. Proc Natl Acad Sci U S A. 2010. PMID: 20534532 Free PMC article.
-
Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans.J Virol. 2007 Oct;81(20):11139-47. doi: 10.1128/JVI.01235-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686867 Free PMC article.
-
Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus.J Infect Dis. 2010 Oct 15;202(8):1161-70. doi: 10.1086/656365. J Infect Dis. 2010. PMID: 20815704 Free PMC article.
-
Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection.J Virol. 2001 Jun;75(11):5141-50. doi: 10.1128/JVI.75.11.5141-5150.2001. J Virol. 2001. PMID: 11333895 Free PMC article.
References
-
- Bale J F, O'Neil M E, Giller R, Perlman S, Koszinowski U. Murine cytomegalovirus genomic material in marrow cells: relation to altered leukocyte counts during sublethal infection of mice. J Infect Dis. 1987;155:207–212. - PubMed
-
- Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. - PubMed
-
- Bonyhadi M L, Rabin L, Sallmi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

