Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6(gag)
- PMID: 10846106
- PMCID: PMC112121
- DOI: 10.1128/jvi.74.13.6198-6202.2000
Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6(gag)
Abstract
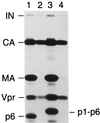
Mature human immunodeficiency virus type 1 (HIV-1) virions contain a typically cone-shaped core that encases the viral genome. In this study, we established conditions which allowed the efficient isolation of morphologically intact HIV-1 cores from virions. The isolated cores consisted mostly of cones which appeared uniformly capped at both ends but were heterogeneous with respect to the shape of the broad cap as well as the dimensions and angle of the cone. Vpr, a nonstructural virion component implicated in the nuclear import of the viral genome, was recovered in core preparations of HIV-1 and simian immunodeficiency viruses from African green monkeys. Unexpectedly, p6(gag), a structural protein required for the incorporation of Vpr, was absent from HIV-1 core preparations. Taken together, our results indicate that the incorporation of Vpr into the virion core is a conserved feature of primate lentiviruses and that the interactions required for the uptake of Vpr into assembling particles differ from those which confine Vpr within the core.
Figures


References
-
- Bolognesi D P, Montelaro R C, Frank H, Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978;199:183–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

