Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex
- PMID: 10848588
- PMCID: PMC85864
- DOI: 10.1128/MCB.20.13.4614-4625.2000
Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex
Abstract
Ubiquitination and subsequent degradation of critical cell cycle regulators is a key mechanism exploited by the cell to ensure an irreversible progression of cell cycle events. The anaphase-promoting complex (APC) is a ubiquitin ligase that targets proteins for degradation by the 26S proteasome. Here we identify the Hsl1p protein kinase as an APC substrate that interacts with Cdc20p and Cdh1p, proteins that mediate APC ubiquitination of protein substrates. Hsl1p is absent in G(1), accumulates as cells begin to bud, and disappears in late mitosis. Hsl1p is stabilized by mutations in CDH1 and CDC23, both of which result in compromised APC activity. Unlike Hsl1p, Gin4p and Kcc4p, protein kinases that have sequence homology to Hsl1p, were stable in G(1)-arrested cells containing active APC. Mutation of a destruction box motif within Hsl1p (Hsl1p(db-mut)) stabilized Hsl1p. Interestingly, this mutation also disrupted the Hsl1p-Cdc20p interaction and reduced the association between Hsl1p and Cdh1p in coimmunoprecipitation studies. These findings suggest that the destruction box motif is required for Cdc20p and, to a lesser extent, for Cdh1p to target Hsl1p to the APC for ubiquitination. Hsl1p has been previously shown to inhibit Swe1p, a protein kinase that negatively regulates the cyclin-dependent kinase Cdc28p, by promoting Swe1p degradation via SCF(Met30) in a bud morphogenesis checkpoint. Results of the present work indicate that Hsl1p is degraded in an APC-dependent manner and suggest a link between the SCF (Skp1-cullin-F box) and APC-proteolytic systems that may help to coordinate the proper progression of cell cycle events.
Figures



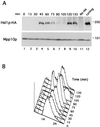

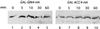

References
-
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
-
- Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355:368–371. - PubMed
-
- Biggins S, Murray A W. Sister chromatid cohesion in mitosis. Curr Opin Cell Biol. 1998;10:769–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
