Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors
- PMID: 10848605
- PMCID: PMC85918
- DOI: 10.1128/MCB.20.13.4791-4805.2000
Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors
Abstract
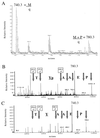
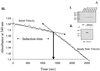
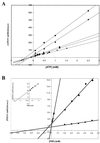
Members of the Eph family of receptor tyrosine kinases exhibit a striking degree of amino acid homology, particularly notable in the kinase and membrane-proximal regions. A mutagenesis approach was taken to address the functions of specific conserved tyrosine residues within these catalytic and juxtamembrane domains. Ligand stimulation of wild-type EphB2 in neuronal NG108-15 cells resulted in an upregulation of catalytic activity and an increase in cellular tyrosine phosphorylation, accompanied by a retraction of neuritic processes. Tyrosine-to-phenylalanine substitutions within the conserved juxtamembrane motif abolished these responses. The mechanistic basis for these observations was examined using the highly related EphA4 receptor in a continuous coupled kinase assay. Tandem mass spectrometry experiments confirmed autophosphorylation of the two juxtamembrane tyrosine residues and also identified a tyrosine within the kinase domain activation segment as a phosphorylation site. Kinetic analysis revealed a decreased affinity for peptide substrate upon substitution of activation segment or juxtamembrane tyrosines. Together, our data suggest that the catalytic and therefore biological activities of Eph receptors are controlled by a two-component inhibitory mechanism, which is released by phosphorylation of the juxtamembrane and activation segment tyrosine residues.
Figures









References
-
- Barker S C, Kassel D B, Weigl D, Huang X, Luther M A, Knight W B. Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry. 1995;34:14843–14851. - PubMed
-
- Baxter R M, Secrist J P, Vaillancourt R R, Kazlauskas A. Full activation of the platelet-derived growth factor beta-receptor kinase involves multiple events. J Biol Chem. 1998;273:17050–17055. - PubMed
-
- Becker N, Seitanidou T, Murphy P, Mattei M G, Topilko P, Nieto M A, Wilkinson D G, Charnay P, Gilardi H P. Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mech Dev. 1994;47:3–17. - PubMed
-
- Bohme B, VandenBos T, Cerretti D P, Park L S, Holtrich U, Rubsamen-Waigmann H, Strebhardt K. Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem. 1996;271:24747–24752. - PubMed
-
- Bruckner K, Pasquale E B, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
