Bypass of a meiotic checkpoint by overproduction of meiotic chromosomal proteins
- PMID: 10848609
- PMCID: PMC85935
- DOI: 10.1128/MCB.20.13.4838-4848.2000
Bypass of a meiotic checkpoint by overproduction of meiotic chromosomal proteins
Abstract
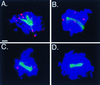
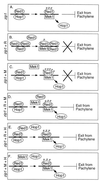
The Saccharomyces cerevisiae zip1 mutant, which exhibits defects in synaptonemal complex formation and meiotic recombination, triggers a checkpoint that causes cells to arrest at the pachytene stage of meiotic prophase. Overproduction of either the meiotic chromosomal protein Red1 or the meiotic kinase Mek1 bypasses this checkpoint, allowing zip1 cells to sporulate. Red1 or Mek1 overproduction also promotes sporulation of other mutants (zip2, dmc1, hop2) that undergo checkpoint-mediated arrest at pachytene. In addition, Red1 overproduction antagonizes interhomolog interactions in the zip1 mutant, substantially decreasing double-strand break formation, meiotic recombination, and homologous chromosome pairing. Mek1 overproduction, in contrast, suppresses checkpoint-induced arrest without significantly decreasing meiotic recombination. Cooverproduction of Red1 and Mek1 fails to bypass the checkpoint; moreover, overproduction of the meiotic chromosomal protein Hop1 blocks the Red1 and Mek1 overproduction phenotypes. These results suggest that meiotic chromosomal proteins function in the signaling of meiotic prophase defects and that the correct stoichiometry of Red1, Mek1, and Hop1 is needed to achieve checkpoint-mediated cell cycle arrest at pachytene.
Figures



References
-
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. - PubMed
-
- Bailis J M, Roeder G S. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell. 2000;101:211–221. - PubMed
-
- Bishop D, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. - PubMed
-
- Bishop D K. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell. 1994;79:1081–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
