Isoform-specific localization of A-RAF in mitochondria
- PMID: 10848612
- PMCID: PMC85938
- DOI: 10.1128/MCB.20.13.4870-4878.2000
Isoform-specific localization of A-RAF in mitochondria
Abstract
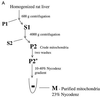
RAF kinase is a family of isoforms including A-RAF, B-RAF, and C-RAF. Despite the important role of RAF in cell growth and proliferation, little evidence exists for isoform-specific function of RAF family members. Using Western analysis and immunogold labeling, A-RAF was selectively localized in highly purified rat liver mitochondria. Two novel human proteins, which interact specifically with A-RAF, were identified, and the full-length sequences are reported. These proteins, referred to as hTOM and hTIM, are similar to components of mitochondrial outer and inner membrane protein-import receptors from lower organisms, implicating their involvement in the mitochondrial transport of A-RAF. hTOM contains multiple tetratricopeptide repeat (TPR) domains, which function in protein-protein interactions. TPR domains are frequently present in proteins involved in cellular transport systems. In contrast, protein 14-3-3, an abundant cytosolic protein that participates in many facets of signal transduction, was found to interact with C-RAF but not with A-RAF N-terminal domain. This information is discussed in view of the important role of mitochondria in cellular functions involving energy balance, proliferation, and apoptosis and the potential role of A-RAF in regulating these systems.
Figures







Similar articles
-
Two isoforms of PSAP/MTCH1 share two proapoptotic domains and multiple internal signals for import into the mitochondrial outer membrane.Am J Physiol Cell Physiol. 2007 Oct;293(4):C1347-61. doi: 10.1152/ajpcell.00431.2006. Epub 2007 Aug 1. Am J Physiol Cell Physiol. 2007. PMID: 17670888
-
Identification of the protein import components of the rat mitochondrial inner membrane, rTIM17, rTIM23, and rTIM44.J Biochem. 1998 Apr;123(4):722-32. doi: 10.1093/oxfordjournals.jbchem.a021997. J Biochem. 1998. PMID: 9538267
-
The RAF family: an expanding network of post-translational controls and protein-protein interactions.Cell Res. 1998 Jun;8(2):81-98. doi: 10.1038/cr.1998.9. Cell Res. 1998. PMID: 9669024 Review.
-
Characterization of rat TOM70 as a receptor of the preprotein translocase of the mitochondrial outer membrane.J Cell Sci. 2002 May 1;115(Pt 9):1895-905. doi: 10.1242/jcs.115.9.1895. J Cell Sci. 2002. PMID: 11956321
-
Mitochondria-targeting sequence, a multi-role sorting sequence recognized at all steps of protein import into mitochondria.J Biochem. 1998 Jun;123(6):1010-6. doi: 10.1093/oxfordjournals.jbchem.a022036. J Biochem. 1998. PMID: 9603986 Review.
Cited by
-
Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection.Antioxidants (Basel). 2024 Apr 23;13(5):504. doi: 10.3390/antiox13050504. Antioxidants (Basel). 2024. PMID: 38790609 Free PMC article. Review.
-
Mitochondrial signaling pathways: a receiver/integrator organelle.Mol Cell Biochem. 2004 Jul;262(1-2):1-16. doi: 10.1023/b:mcbi.0000038228.85494.3b. Mol Cell Biochem. 2004. PMID: 15532704 Review.
-
Conformation-specific inhibitors of activated Ras GTPases reveal limited Ras dependency of patient-derived cancer organoids.J Biol Chem. 2020 Apr 3;295(14):4526-4540. doi: 10.1074/jbc.RA119.011025. Epub 2020 Feb 20. J Biol Chem. 2020. PMID: 32086379 Free PMC article.
-
Ras, an actor on many stages: posttranslational modifications, localization, and site-specified events.Genes Cancer. 2011 Mar;2(3):182-94. doi: 10.1177/1947601911409213. Genes Cancer. 2011. PMID: 21779492 Free PMC article.
-
Spatial regulation of ARAF controls the MST2-Hippo pathway.Small GTPases. 2019 Jul;10(4):243-248. doi: 10.1080/21541248.2017.1288686. Epub 2017 Mar 10. Small GTPases. 2019. PMID: 28281933 Free PMC article.
References
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1992.
-
- Avruch J, Zhang X, Kyriakis J M. Raf meets Ras: completing the framework of signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
