Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells
- PMID: 10848634
- PMCID: PMC14908
- DOI: 10.1091/mbc.11.6.2131
Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells
Abstract
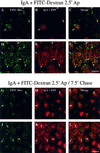
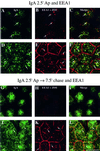
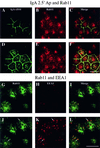
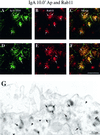
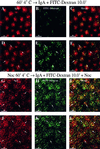
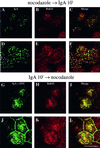
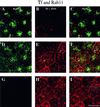
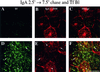
When fluid-phase markers are internalized from opposite poles of polarized Madin-Darby canine kidney cells, they accumulate in distinct apical and basolateral early endosomes before meeting in late endosomes. Recent evidence suggests that significant mixing of apically and basolaterally internalized membrane proteins occurs in specialized apical endosomal compartments, including the common recycling endosome and the apical recycling endosome (ARE). The relationship between these latter compartments and the fluid-labeled apical early endosome is unknown at present. We report that when the apical recycling marker, membrane-bound immunoglobulin A (a ligand for the polymeric immunoglobulin receptor), and fluid-phase dextran are cointernalized from the apical poles of Madin-Darby canine kidney cells, they enter a shared apical early endosome (</=2.5 min at 37 degrees C) and are then rapidly segregated from one another. The dextran remains in the large supranuclear EEA1-positive early endosomes while recycling polymeric immunoglobulin receptor-bound immunoglobulin A is delivered to a Rab11-positive subapical recycling compartment. This latter step requires an intact microtubule cytoskeleton. Receptor-bound transferrin, a marker of the basolateral recycling pathway, has limited access to the fluid-rich apical early endosome but is excluded from the subapical elements of the Rab11-positive recycling compartment. We propose that the term ARE be used to describe the subapical Rab11-positive compartment and that the ARE is distinct from both the transferrin-rich common recycling endosome and the fluid-rich apical early endosome.
Figures


Similar articles
-
Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells.Mol Biol Cell. 1999 Jan;10(1):47-61. doi: 10.1091/mbc.10.1.47. Mol Biol Cell. 1999. PMID: 9880326 Free PMC article.
-
Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling.Traffic. 2000 Feb;1(2):124-40. doi: 10.1034/j.1600-0854.2000.010205.x. Traffic. 2000. PMID: 11208093
-
Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome.J Cell Biol. 1994 Jan;124(1-2):83-100. doi: 10.1083/jcb.124.1.83. J Cell Biol. 1994. PMID: 7905002 Free PMC article.
-
The subapical compartment: a traffic center in membrane polarity development.J Cell Sci. 2004 May 1;117(Pt 11):2183-92. doi: 10.1242/jcs.01217. J Cell Sci. 2004. PMID: 15126620 Review.
-
Early Endosome Morphology in Health and Disease.Adv Exp Med Biol. 2018;1074:335-343. doi: 10.1007/978-3-319-75402-4_41. Adv Exp Med Biol. 2018. PMID: 29721961 Free PMC article. Review.
Cited by
-
Rab11a-positive compartments in proximal tubule cells sort fluid-phase and membrane cargo.Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C441-9. doi: 10.1152/ajpcell.00236.2013. Epub 2013 Oct 23. Am J Physiol Cell Physiol. 2014. PMID: 24153428 Free PMC article.
-
Generation of Rab-based transgenic lines for in vivo studies of endosome biology in zebrafish.Dev Dyn. 2011 Nov;240(11):2452-65. doi: 10.1002/dvdy.22758. Epub 2011 Oct 4. Dev Dyn. 2011. PMID: 21976318 Free PMC article.
-
The fast-recycling receptor Megalin defines the apical recycling pathway of epithelial cells.Nat Commun. 2016 May 16;7:11550. doi: 10.1038/ncomms11550. Nat Commun. 2016. PMID: 27180806 Free PMC article.
-
Transformation of rat intestinal epithelial cells by overexpression of Rab25 is microtubule dependent.Cytoskeleton (Hoboken). 2011 Feb;68(2):97-111. doi: 10.1002/cm.20497. Epub 2011 Jan 3. Cytoskeleton (Hoboken). 2011. PMID: 21246754 Free PMC article.
-
Transcytosis of polymeric immunoglobulin a in polarized Madin-Darby canine kidney cells.Methods Mol Biol. 2008;440:157-70. doi: 10.1007/978-1-59745-178-9_12. Methods Mol Biol. 2008. PMID: 18369944 Free PMC article.
References
-
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. - PubMed
-
- Breitfeld P, Casanova JE, Harris JM, Simister NE, Mostov KE. Expression and analysis of the polymeric immunoglobulin receptor. Methods Cell Biol. 1989a;32:329–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

