Elo1p-dependent carboxy-terminal elongation of C14:1Delta(9) to C16:1Delta(11) fatty acids in Saccharomyces cerevisiae
- PMID: 10850979
- PMCID: PMC94535
- DOI: 10.1128/JB.182.13.3655-3660.2000
Elo1p-dependent carboxy-terminal elongation of C14:1Delta(9) to C16:1Delta(11) fatty acids in Saccharomyces cerevisiae
Abstract
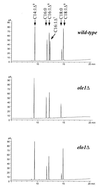
Saccharomyces cerevisiae medium-chain acyl elongase (ELO1) mutants have previously been isolated in screens for fatty acid synthetase (FAS) mutants that fail to grow on myristic acid (C14:0)-supplemented media. Here we report that wild-type cells cultivated in myristoleic acid (C14:1Delta(9))-supplemented media synthesized a novel unsaturated fatty acid that was identified as C16:1Delta(11) fatty acid by gas chromatography-mass spectroscopy. Synthesis of C16:1Delta(11) was dependent on a functional ELO1 gene, indicating that Elo1p catalyzes carboxy-terminal elongation of unsaturated fatty acids (alpha-elongation). In wild-type cells, the C16:1Delta(11) elongation product accounted for approximately 12% of the total fatty acids. This increased to 18% in cells that lacked a functional acyl chain desaturase (ole1Delta mutants) and hence were fully dependent on uptake and elongation of C14:1. The observation that ole1Delta mutant cells grew almost like wild type on medium supplemented with C14:1 indicated that uptake and elongation of unsaturated fatty acids were efficient. Interestingly, wild-type cells supplemented with either C14:1 or C16:1 fatty acids displayed dramatic alterations in their phospholipid composition, suggesting that the availability of acyl chains is a dominant determinant of the phospholipid class composition of cellular membranes. In particular, the relative content of the two major phospholipid classes, phosphatidylethanolamine and phosphatidylcholine, was strongly dependent on the chain length of the supplemented fatty acid. Moreover, analysis of the acyl chain composition of individual phospholipid classes in cells supplemented with C14:1 revealed that the relative degree of acyl chain saturation characteristic for each phospholipid class appeared to be conserved, despite the gross alteration in the cellular acyl chain pool. Comparison of the distribution of fatty acids that were taken up and elongated (C16:1Delta(11)) to those that were endogenously synthesized by fatty acid synthetase and then desaturated by Ole1p (C16:1Delta(9)) in individual phospholipid classes finally suggested the presence of two different pools of diacylglycerol species. These results will be discussed in terms of biosynthesis of different phospholipid classes via either the de novo or the Kennedy pathway.
Figures
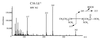
References
-
- Bloom M, Evans E, Mouritsen O G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991;24:293–397. - PubMed
-
- Broekhuyse R M. Phospholipids in tissues of the eye. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl-ether phospholipids. Biochim Biophys Acta. 1968;260:449–459. - PubMed
-
- Browse J, Somerville C. Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

