Physical morphology and surface properties of unsaturated Pseudomonas putida biofilms
- PMID: 10850998
- PMCID: PMC94554
- DOI: 10.1128/JB.182.13.3809-3815.2000
Physical morphology and surface properties of unsaturated Pseudomonas putida biofilms
Abstract
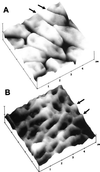
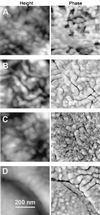
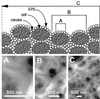
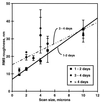
Unsaturated biofilms of Pseudomonas putida, i.e., biofilms grown in humid air, were analyzed by atomic force microscopy to determine surface morphology, roughness, and adhesion forces in the outer and basal cell layers of fresh and desiccated biofilms. Desiccated biofilms were equilibrated with a 75.5% relative humidity atmosphere, which is far below the relative humidity of 98 to 99% at which these biofilms were cultured. In sharp contrast to the effects of drying on biofilms grown in fluid, we observed that drying caused little change in morphology, roughness, or adhesion forces in these unsaturated biofilms. Surface roughness for moist and dry biofilms increased approximately linearly with increasing scan sizes. This indicated that the divides between bacteria contributed more to overall roughness than did extracellular polymeric substances (EPS) on individual bacteria. The EPS formed higher-order structures we termed mesostructures. These mesostructures are much larger than the discrete polymers of glycolipids and proteins that have been previously characterized on the outer surface of these gram-negative bacteria.
Figures


References
-
- Arnoldi M, Kacher C M, Baeuerlein E, Radmacher M, Fritz M. Elastic properties of the cell wall of Magnetospirillum gryphiswaldense investigated by atomic force microscopy. Appl Phys A. 1998;66:S613–S617.
-
- Beech I B. The potential use of atomic force microscopy for studying corrosion of metals in the presence of bacterial biofilms—an overview. Int Biodeterior Biodegrad. 1996;37:141–149.
-
- Bishop P L, Gibbs J T, Cunningham B E. Relationship between concentration and hydrodynamic boundary layers over biofilms. Environ Technol. 1997;18:375–386.
-
- Bremer P J, Geesey G G, Drake B. Atomic force microscopy examination of the topography of a hydrated bacterial biofilm on a copper surface. Curr Microbiol. 1992;24:223–230.
-
- Bustamante C, Keller D. Scanning force microscopy in biology. Phys Today. 1995;48:32–38.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

